Participatory methods used in the evaluation of medical devices: a comparison of focus groups, interviews, and a survey
- PMID: 38609933
- PMCID: PMC11015660
- DOI: 10.1186/s12913-024-10887-3
Participatory methods used in the evaluation of medical devices: a comparison of focus groups, interviews, and a survey
Abstract
Background: Stakeholder engagement in evaluation of medical devices is crucial for aligning devices with stakeholders' views, needs, and values. Methods for these engagements have however not been compared to analyse their relative merits for medical device evaluation. Therefore, we systematically compared these three methods in terms of themes, interaction, and time-investment.
Methods: We compared focus groups, interviews, and an online survey in a case-study on minimally invasive endoscopy-guided surgery for patients with intracerebral haemorrhage. The focus groups and interviews featured two rounds, one explorative focussing on individual perspectives, and one interactive focussing on the exchange of perspectives between participants. The comparison between methods was made in terms of number and content of themes, how participants interact, and hours invested by all researchers.
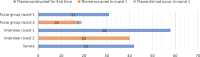
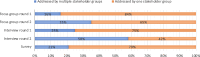
Results: The focus groups generated 34 themes, the interviews 58, and the survey 42. Various improvements for the assessment of the surgical procedure were only discussed in the interviews. In focus groups, participants were inclined to emphasise agreement and support, whereas the interviews consisted of questions and answers. The total time investment for researchers of focus groups was 95 h, of interviews 315 h, and survey 81 h.
Conclusions: Within the context of medical device evaluation, interviews appeared to be the most appropriate method for understanding stakeholder views since they provide a scope and depth of information that is not generated by other methods. Focus groups were useful to rapidly bring views together. Surveys enabled a quick exploration. Researchers should account for these methodological differences and select the method that is suitable for their research aim.
Keywords: Comparison; Medical devices; Neurology; Qualitative methods; Stakeholder involvement; Surgery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Royal College of Surgeons. Future Surg. 2022 [cited 2022 Jul 14]. https://futureofsurgery.rcseng.ac.uk/#start. Accessed 4 April 2024.
-
- Food and Drug Administration (FDA) Patient Engagement in the Design and Conduct of Medical Device Clinical Studies Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders. 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed 4 April 2024.
-
- The European Parliament and the Council of the European Union Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. http://data.europa.eu/eli/reg/2017/745/oj. Accessed 4 April 2024.
-
- Bitkina OV, Kim HK, Park J. Usability and user experience of medical devices: an overview of the current state, analysis methodologies, and future challenges. Int J Ind Ergon. 2020;76(November 2019):102932. doi: 10.1016/j.ergon.2020.102932. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

