Urate-lowering therapy, serum urate, inflammatory biomarkers, and renal function in patients with gout following pegloticase discontinuation
- PMID: 38609967
- PMCID: PMC11010378
- DOI: 10.1186/s13075-024-03318-5
Urate-lowering therapy, serum urate, inflammatory biomarkers, and renal function in patients with gout following pegloticase discontinuation
Abstract
Background/purpose: Little is known about long-term clinical outcomes or urate-lowering (ULT) therapy use following pegloticase discontinuation. We examined ULT use, serum urate (SU), inflammatory biomarkers, and renal function following pegloticase discontinuation.
Methods: We conducted a retrospective analysis of gout patients who discontinued pegloticase using the Rheumatology Informatics System for Effectiveness (RISE) registry from 1/2016 to 6/2022. We defined discontinuation as a gap ≥ 12 weeks after last infusion. We examined outcomes beginning two weeks after last dose and identified ULT therapy following pegloticase discontinuation. We evaluated changes in lab values (SU, eGFR, CRP and ESR), comparing on- treatment (≤ 15 days of the second pegloticase dose) to post-treatment.
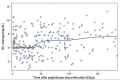
Results: Of the 375 gout patients discontinuing pegloticase, median (IQR) laboratory changes following discontinuation were: SU: +2.4 mg/dL (0.0,6.3); eGFR: -1.9 mL/min (- 8.7,3.7); CRP: -0.8 mg/L (-12.8,0.0); and ESR: -4.0 mm/hr (-13.0,0.0). Therapy post-discontinuation included oral ULTs (86.0%), restarting pegloticase (4.5%), and no documentation of ULT (9.5%), excluding patients with multiple same-day prescriptions (n = 17). Oral ULTs following pegloticase were: 62.7% allopurinol, 34.1% febuxostat. The median (IQR) time to starting/restarting ULT was 92.0 days (55.0,173.0). Following ULT prescribing (≥ 30 days), only 51.0% of patients had SU < 6 mg/dL. Patients restarting pegloticase achieved a median SU of 0.9 mg/dL (IQR:0.2,9.7) and 58.3% had an SU < 6 mg/dL.
Conclusion: Pegloticase treats uncontrolled gout in patients with failed response to xanthine oxidase inhibitors, but among patients who discontinue, optimal treatment is unclear. Based on this analysis, only half of those starting another ULT achieved target SU. Close follow-up is needed to optimize outcomes after pegloticase discontinuation.
Keywords: Discontinuation; Gout; Pegloticase; Restart; Treatment gap.
© 2024. The Author(s).
Conflict of interest statement
EEH, ASM, FX, and JZ declare no conflicts of interest. TRM receives support from Elsevier, UpToDate, Horizon (now Amgen Inc.), Pfizer, Sanofi, UCB, and the Rheumatology Research Foundation. BL and LPS are employees of and stockholders in Horizon Therapeutics (now Amgen Inc.). JRC receives support for unrelated work from AbbVie, Amgen, Aqtual, Bendcare, BMS, CorEvitas, FASTER, GSK, Janssen, Lilly, Moderna, Novartis, Pfizer, Sanofi, Scipher, Setpoint, TNacity Blue Ocean, and UCB.
Figures



References
-
- Hammam N, Li J, Kay J, Izadi Z, Yazdany J, Schmajuk G. Monitoring and achievement of target serum urate among gout patients receiving long-term urate‐lowering therapy in the American College of Rheumatology RISE Registry. Arthritis Care Res (Hoboken) 2023;75(7):1544–52. doi: 10.1002/acr.25009. - DOI - PMC - PubMed
-
- Botson JK, Saag K, Peterson J, Parikh N, Ong S, La D, et al. A randomized, placebo-controlled study of methotrexate to increase response rates in patients with uncontrolled gout receiving pegloticase: primary efficacy and safety findings. Arthritis Rheumatol. 2023;75(2):293–304. doi: 10.1002/art.42335. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

