PREX2 contributes to radiation resistance by inhibiting radiotherapy-induced tumor immunogenicity via cGAS/STING/IFNs pathway in colorectal cancer
- PMID: 38609982
- PMCID: PMC11015576
- DOI: 10.1186/s12916-024-03375-2
PREX2 contributes to radiation resistance by inhibiting radiotherapy-induced tumor immunogenicity via cGAS/STING/IFNs pathway in colorectal cancer
Erratum in
-
Correction: PREX2 contributes to radiation resistance by inhibiting radiotherapy-induced tumor immunogenicity via cGAS/STING/IFNs pathway in colorectal cancer.BMC Med. 2025 Aug 9;23(1):465. doi: 10.1186/s12916-025-04331-4. BMC Med. 2025. PMID: 40781623 Free PMC article. No abstract available.
Abstract
Background: Colorectal cancer (CRC) lacks established biomarkers or molecular targets for predicting or enhancing radiation response. Phosphatidylinositol-3,4,5-triphosphate-dependent Rac exchange factor 2 (PREX2) exhibits intricate implications in tumorigenesis and progression. Nevertheless, the precise role and underlying mechanisms of PREX2 in CRC radioresistance remain unclear.
Methods: RNA-seq was employed to identify differentially expressed genes between radioresistant CRC cell lines and their parental counterparts. PREX2 expression was scrutinized using Western blotting, real-time PCR, and immunohistochemistry. The radioresistant role of PREX2 was assessed through in vitro colony formation assay, apoptosis assay, comet assay, and in vivo xenograft tumor models. The mechanism of PREX2 was elucidated using RNA-seq and Western blotting. Finally, a PREX2 small-molecule inhibitor, designated PREX-in1, was utilized to enhance the efficacy of ionizing radiation (IR) therapy in CRC mouse models.
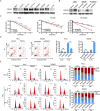
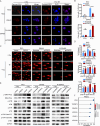
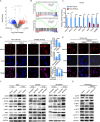
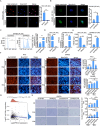
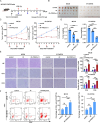
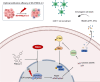
Results: PREX2 emerged as the most significantly upregulated gene in radioresistant CRC cells. It augmented the radioresistant capacity of CRC cells and demonstrated potential as a marker for predicting radioresistance efficacy. Mechanistically, PREX2 facilitated DNA repair by upregulating DNA-PKcs, suppressing radiation-induced immunogenic cell death, and impeding CD8+ T cell infiltration through the cGAS/STING/IFNs pathway. In vivo, the blockade of PREX2 heightened the efficacy of IR therapy.
Conclusions: PREX2 assumes a pivotal role in CRC radiation resistance by inhibiting the cGAS/STING/IFNs pathway, presenting itself as a potential radioresistant biomarker and therapeutic target for effectively overcoming radioresistance in CRC.
Keywords: Colorectal cancer; Immunogenic cell death; PREX2; Radioresistance; cGAS/STING/IFNs.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44(5):1027–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2021YFF1201004/the National Key R&D Program of China
- 2023A04J2383/the Guangzhou Basic and Applied Basic Research Topics
- Grant No.82273358/the National Natural Science Foundation of China
- 82003059/the National Natural Science Foundation of China
- No.81872041/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

