The World Health Organization Antenatal CorTicosteroids for Improving Outcomes in preterm Newborns (ACTION-III) Trial: study protocol for a multi-country, multi-centre, double-blind, three-arm, placebo-controlled, individually randomized trial of antenatal corticosteroids for women at high probability of late preterm birth in hospitals in low- resource countries
- PMID: 38609983
- PMCID: PMC11010373
- DOI: 10.1186/s13063-024-07941-0
The World Health Organization Antenatal CorTicosteroids for Improving Outcomes in preterm Newborns (ACTION-III) Trial: study protocol for a multi-country, multi-centre, double-blind, three-arm, placebo-controlled, individually randomized trial of antenatal corticosteroids for women at high probability of late preterm birth in hospitals in low- resource countries
Abstract
Background: Preterm birth complications are the leading cause of newborn and under-5 mortality. Over 85% of all preterm births occur in the late preterm period, i.e. between 34 and < 37 weeks of gestation. Antenatal corticosteroids (ACS) prevent mortality and respiratory morbidity when administered to women at high risk of an early preterm birth, i.e. < 34 weeks' gestation. However, the benefits and risks of ACS in the late preterm period are less clear; both guidelines and practices vary between settings. Emerging evidence suggests that the benefits of ACS may be achievable at lower doses than presently used. This trial aims to determine the efficacy and safety of two ACS regimens compared to placebo, when given to women with a high probability of late preterm birth, in hospitals in low-resource countries.
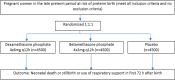
Methods: WHO ACTION III trial is a parallel-group, three-arm, individually randomized, double-blind, placebo-controlled trial of two ACS regimens: dexamethasone phosphate 4 × 6 mg q12h or betamethasone phosphate 4 × 2 mg q 12 h. The trial is being conducted across seven sites in five countries-Bangladesh, India, Kenya, Nigeria, and Pakistan. Eligible women are those with a gestational age between 34 weeks 0 days and 36 weeks 5 days, who have a high probability of preterm birth between 12 h and 7 days (up to 36 weeks 6 days gestation). The primary outcome is a composite of stillbirth or neonatal death within 72 h of birth or use of newborn respiratory support within 72 h of birth or prior to discharge from hospital, whichever is earlier. Secondary outcomes include safety and health utilization measures for both women and newborns. The sample size is 13,500 women.
Discussion: This trial will evaluate the benefits and possible harms of ACS when used in women likely to have a late preterm birth. It will also evaluate a lower-dose ACS regimen based on literature from pharmacokinetic studies. The results of this trial will provide robust critical evidence on the safe and appropriate use of ACS in the late preterm period internationally.
Trial registration: ISRCTN11434567 . Registered on 7 June 2021.
Keywords: Antenatal corticosteroids; Betamethasone; Dexamethasone; Late preterm birth; Low- resource setting.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels & Trends in Child Mortality: Report 2022, Estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York: United Nations Children’s Fund; 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

