Peripheral extracellular vesicles in neurodegeneration: pathogenic influencers and therapeutic vehicles
- PMID: 38610012
- PMCID: PMC11015679
- DOI: 10.1186/s12951-024-02428-1
Peripheral extracellular vesicles in neurodegeneration: pathogenic influencers and therapeutic vehicles
Abstract
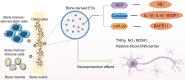
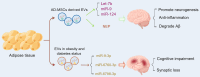
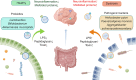
Neurodegenerative diseases (NDDs) such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis epitomize a class of insidious and relentless neurological conditions that are difficult to cure. Conventional therapeutic regimens often fail due to the late onset of symptoms, which occurs well after irreversible neurodegeneration has begun. The integrity of the blood-brain barrier (BBB) further impedes efficacious drug delivery to the central nervous system, presenting a formidable challenge in the pharmacological treatment of NDDs. Recent scientific inquiries have shifted focus toward the peripheral biological systems, investigating their influence on central neuropathology through the lens of extracellular vesicles (EVs). These vesicles, distinguished by their ability to breach the BBB, are emerging as dual operatives in the context of NDDs, both as conveyors of pathogenic entities and as prospective vectors for therapeutic agents. This review critically summarizes the burgeoning evidence on the role of extracerebral EVs, particularly those originating from bone, adipose tissue, and gut microbiota, in modulating brain pathophysiology. It underscores the duplicity potential of peripheral EVs as modulators of disease progression and suggests their potential as novel vehicles for targeted therapeutic delivery, positing a transformative impact on the future landscape of NDD treatment strategies. Search strategy A comprehensive literature search was conducted using PubMed, Web of Science, and Scopus from January 2000 to December 2023. The search combined the following terms using Boolean operators: "neurodegenerative disease" OR "Alzheimer's disease" OR "Parkinson's disease" OR "Amyotrophic lateral sclerosis" AND "extracellular vesicles" OR "exosomes" OR "outer membrane vesicles" AND "drug delivery systems" AND "blood-brain barrier". MeSH terms were employed when searching PubMed to refine the results. Studies were included if they were published in English, involved human subjects, and focused on the peripheral origins of EVs, specifically from bone, adipose tissue, and gut microbiota, and their association with related diseases such as osteoporosis, metabolic syndrome, and gut dysbiosis. Articles were excluded if they did not address the role of EVs in the context of NDDs or did not discuss therapeutic applications. The titles and abstracts of retrieved articles were screened using a dual-review process to ensure relevance and accuracy. The reference lists of selected articles were also examined to identify additional relevant studies.
Keywords: Blood-brain barrier; Extracellular vesicles; Neurodegenerative disease; Peripheral-brain axis; Therapeutic delivery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Extracellular Vesicles in the Treatment of Parkinson's Disease: A Review.Curr Med Chem. 2021;28(31):6375-6394. doi: 10.2174/0929867328666210113170941. Curr Med Chem. 2021. PMID: 33441061 Review.
-
Extracellular vesicles, the emerging mirrors of brain physiopathology.Int J Biol Sci. 2023 Jan 1;19(3):721-743. doi: 10.7150/ijbs.79063. eCollection 2023. Int J Biol Sci. 2023. PMID: 36778117 Free PMC article. Review.
-
Role of extracellular vesicles in neurodegenerative diseases.Prog Neurobiol. 2021 Jun;201:102022. doi: 10.1016/j.pneurobio.2021.102022. Epub 2021 Feb 19. Prog Neurobiol. 2021. PMID: 33617919 Review.
-
Research progress on the role of extracellular vesicles in neurodegenerative diseases.Transl Neurodegener. 2023 Sep 11;12(1):43. doi: 10.1186/s40035-023-00375-9. Transl Neurodegener. 2023. PMID: 37697342 Free PMC article. Review.
-
Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders.J Control Release. 2020 Jul 10;323:225-239. doi: 10.1016/j.jconrel.2020.04.017. Epub 2020 Apr 11. J Control Release. 2020. PMID: 32289328 Free PMC article. Review.
Cited by
-
Antiageing strategy for neurodegenerative diseases: from mechanisms to clinical advances.Signal Transduct Target Ther. 2025 Mar 10;10(1):76. doi: 10.1038/s41392-025-02145-7. Signal Transduct Target Ther. 2025. PMID: 40059211 Free PMC article. Review.
-
Inhibition of tRF- 02514 in Extracellular Vesicles Preserves Microglia Pyroptosis and Protects Against Parkinson's Disease.Mol Neurobiol. 2025 Sep;62(9):11047-11063. doi: 10.1007/s12035-025-04925-2. Epub 2025 Apr 21. Mol Neurobiol. 2025. PMID: 40254704 Free PMC article.
-
Brown Adipose Tissue undergoes pathological perturbations and shapes C2C12 myoblast homeostasis in the SOD1-G93A mouse model of Amyotrophic Lateral Sclerosis.Heliyon. 2025 Jan 23;11(3):e41801. doi: 10.1016/j.heliyon.2025.e41801. eCollection 2025 Feb 15. Heliyon. 2025. PMID: 39916853 Free PMC article.
-
Bone-brain interaction: mechanisms and potential intervention strategies of biomaterials.Bone Res. 2025 Mar 17;13(1):38. doi: 10.1038/s41413-025-00404-5. Bone Res. 2025. PMID: 40097409 Free PMC article. Review.
-
Gut microbiota linked to hydrocephalus through inflammatory factors: a Mendelian randomization study.Front Immunol. 2024 Jul 15;15:1372051. doi: 10.3389/fimmu.2024.1372051. eCollection 2024. Front Immunol. 2024. PMID: 39076985 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- 82301625; 81801395; 82125023; 82072504; 81871822; U22A20300, 81971029/National Natural Science Foundation of China
- 82301625; 81801395; 82125023; 82072504; 81871822; U22A20300, 81971029/National Natural Science Foundation of China
- 82301625; 81801395; 82125023; 82072504; 81871822; U22A20300, 81971029/National Natural Science Foundation of China
- 82301625; 81801395; 82125023; 82072504; 81871822; U22A20300, 81971029/National Natural Science Foundation of China
- 2020YFC2008500/National Key R&D Program of China
LinkOut - more resources
Full Text Sources
Medical

