Augmented ERO1α upon mTORC1 activation induces ferroptosis resistance and tumor progression via upregulation of SLC7A11
- PMID: 38610018
- PMCID: PMC11015652
- DOI: 10.1186/s13046-024-03039-2
Augmented ERO1α upon mTORC1 activation induces ferroptosis resistance and tumor progression via upregulation of SLC7A11
Abstract
Background: The dysregulated mechanistic target of rapamycin complex 1 (mTORC1) signaling plays a critical role in ferroptosis resistance and tumorigenesis. However, the precise underlying mechanisms still need to be fully understood.
Methods: Endoplasmic reticulum oxidoreductase 1 alpha (ERO1α) expression in mTORC1-activated mouse embryonic fibroblasts, cancer cells, and laryngeal squamous cell carcinoma (LSCC) clinical samples was examined by quantitative real-time PCR (qRT-PCR), western blotting, immunofluorescence (IF), and immunohistochemistry. Extensive in vitro and in vivo experiments were carried out to determine the role of ERO1α and its downstream target, member 11 of the solute carrier family 7 (SLC7A11), in mTORC1-mediated cell proliferation, angiogenesis, ferroptosis resistance, and tumor growth. The regulatory mechanism of ERO1α on SLC7A11 was investigated via RNA-sequencing, a cytokine array, an enzyme-linked immunosorbent assay, qRT-PCR, western blotting, IF, a luciferase reporter assay, and a chromatin immunoprecipitation assay. The combined therapeutic effect of ERO1α inhibition and the ferroptosis inducer imidazole ketone erastin (IKE) on mTORC1-activated cells was evaluated using cell line-derived xenografts, LSCC organoids, and LSCC patient-derived xenograft models.
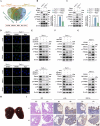
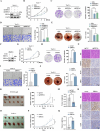
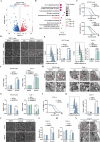
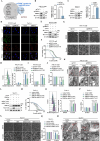
Results: ERO1α is a functional downstream target of mTORC1. Elevated ERO1α induced ferroptosis resistance and exerted pro-oncogenic roles in mTORC1-activated cells via upregulation of SLC7A11. Mechanically, ERO1α stimulated the transcription of SLC7A11 by activating the interleukin-6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) pathway. Moreover, ERO1α inhibition combined with treatment using the ferroptosis inducer IKE exhibited synergistic antitumor effects on mTORC1-activated tumors.
Conclusions: The ERO1α/IL-6/STAT3/SLC7A11 pathway is crucial for mTORC1-mediated ferroptosis resistance and tumor growth, and combining ERO1α inhibition with ferroptosis inducers is a novel and effective treatment for mTORC1-related tumors.
Keywords: ERO1α; Ferroptosis; SLC7A11; Tumor growth; mTOR.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflict of interests to declare
Figures


Similar articles
-
Long non-coding RNA NORAD suppresses erastin-induced ferroptosis in breast cancer by upregulating SLC7A11 via targeting the FUS/NR3C1 axis: experimental studies.Int J Surg. 2025 Jul 1;111(7):4295-4313. doi: 10.1097/JS9.0000000000002531. Epub 2025 May 20. Int J Surg. 2025. PMID: 40405780
-
Tanshinone IIA induces ferroptosis in colorectal cancer cells through the suppression of SLC7A11 expression via the PI3K/AKT/mTOR pathway.Eur J Med Res. 2025 Jul 5;30(1):576. doi: 10.1186/s40001-025-02842-7. Eur J Med Res. 2025. PMID: 40618168 Free PMC article.
-
The mechanism of lncRNA SSTR5-AS1 promoting ferroptosis resistance and immune escape in ovarian cancer cells by recruiting STAT3 to regulate SLC7A11 expression.Cancer Genet. 2025 Sep;296-297:172-181. doi: 10.1016/j.cancergen.2025.07.009. Epub 2025 Jul 16. Cancer Genet. 2025. PMID: 40716132
-
Exosomal lncRNA ROR1-AS1 from cancer-associated fibroblasts inhibits ferroptosis of lung cancer cells through the IGF2BP1/SLC7A11 signal axis.Cell Signal. 2024 Aug;120:111221. doi: 10.1016/j.cellsig.2024.111221. Epub 2024 May 8. Cell Signal. 2024. PMID: 38729321
-
PRKAA2 Promotes Tumor Growth and Inhibits Ferroptosis through SLC7A11/GSH/GPX4 Pathway in Non-Small Cell Lung Cancer.Biotechnol Appl Biochem. 2025 Aug;72(4):954-962. doi: 10.1002/bab.2710. Epub 2024 Dec 25. Biotechnol Appl Biochem. 2025. PMID: 39722125
Cited by
-
SLC16A1 Inhibits Ferroptosis and Promotes the Progression of Head and Neck Squamous Cell Carcinoma.J Cancer. 2025 Mar 29;16(7):2184-2196. doi: 10.7150/jca.110217. eCollection 2025. J Cancer. 2025. PMID: 40302798 Free PMC article.
-
Dicoumarol sensitizes hepatocellular carcinoma cells to ferroptosis induced by imidazole ketone erastin.Front Immunol. 2025 Feb 11;16:1531874. doi: 10.3389/fimmu.2025.1531874. eCollection 2025. Front Immunol. 2025. PMID: 40007539 Free PMC article.
-
CircTTC13 promotes sorafenib resistance in hepatocellular carcinoma through the inhibition of ferroptosis by targeting the miR-513a-5p/SLC7A11 axis.Mol Cancer. 2025 Jan 27;24(1):32. doi: 10.1186/s12943-024-02224-3. Mol Cancer. 2025. PMID: 39871338 Free PMC article.
-
The Role of Cancer Organoids in Ferroptosis, Pyroptosis, and Necroptosis: Functions and Clinical Implications.Biomolecules. 2025 May 2;15(5):659. doi: 10.3390/biom15050659. Biomolecules. 2025. PMID: 40427552 Free PMC article. Review.
-
SLC7A11-mediated cell death mechanism in cancer: a comparative study of disulfidptosis and ferroptosis.Front Cell Dev Biol. 2025 Jun 4;13:1559423. doi: 10.3389/fcell.2025.1559423. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40535572 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 81372475/The Natural Science Foundation of China
- 82171127/The Natural Science Foundation of China
- 82303021/The Natural Science Foundation of China
- 2208085MH239/Anhui Provincial Natural Science Foundation
- KJ2019A0219/Natural Science Foundation of Universities of Anhui Province
- No. 4245/Discipline Construction Project of the First Affiliated Hospital of Anhui Medical University
- 2020xkjT027/2020 Basic and Clinical Cooperative Research Promotion Program of Anhui Medical University
- by2023058/Anhui Medical University Affiliated Bozhou Hospital Talent Introduction Research Initiation Fund
- YZJJ2022QN48/ASHIPS Director's Fund
- YJS20230027/Postgraduate Innovation Research and Practice Program of Anhui Medical University
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

