Nigrostriatal degeneration determines dynamics of glial inflammatory and phagocytic activity
- PMID: 38610019
- PMCID: PMC11015575
- DOI: 10.1186/s12974-024-03091-x
Nigrostriatal degeneration determines dynamics of glial inflammatory and phagocytic activity
Abstract
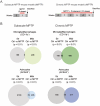
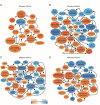
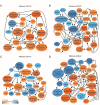
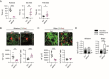
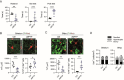
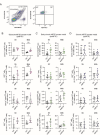
Glial cells are key players in the initiation of innate immunity in neurodegeneration. Upon damage, they switch their basal activation state and acquire new functions in a context and time-dependent manner. Since modulation of neuroinflammation is becoming an interesting approach for the treatment of neurodegenerative diseases, it is crucial to understand the specific contribution of these cells to the inflammatory reaction and to select experimental models that recapitulate what occurs in the human disease. Previously, we have characterized a region-specific activation pattern of CD11b+ cells and astrocytes in the α-synuclein overexpression mouse model of Parkinson´s disease (PD). In this study we hypothesized that the time and the intensity of dopaminergic neuronal death would promote different glial activation states. Dopaminergic degeneration was induced with two administration regimens of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), subacute (sMPTP) and chronic (cMPTP). Our results show that in the sMPTP mouse model, the pro-inflammatory phenotype of striatal CD11b+ cells was counteracted by an anti-inflammatory astrocytic profile. In the midbrain the roles were inverted, CD11b+ cells exhibited an anti-inflammatory profile and astrocytes were pro-inflammatory. The overall response generated resulted in decreased CD4 T cell infiltration in both regions. Chronic MPTP exposure resulted in a mild and prolonged neuronal degeneration that generated a pro-inflammatory response and increased CD4 T cell infiltration in both regions. At the onset of the neurodegenerative process, microglia and astrocytes cooperated in the removal of dopaminergic terminals. With time, only microglia maintained the phagocytic activity. In the ventral midbrain, astrocytes were the main phagocytic mediators at early stages of degeneration while microglia were the major phagocytic cells in the chronic state. In this scenario, we questioned which activation pattern recapitulates better the features of glial activation in PD. Glial activation in the cMPTP mouse model reflects many pathways of their corresponding counterparts in the human brain with advanced PD. Altogether, our results point toward a context-dependent cooperativity of microglia/myeloid cells and astrocytes in response to neuronal damage and the relevance of selecting the right experimental models for the study of neuroinflammation.
Keywords: Microglia; Neurodegeneration; Parkinson´s disease; Phagocytosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
- PI20/01063/Instituto de Salud Carlos III
- PI20/01063/Instituto de Salud Carlos III
- PI20/01063/Instituto de Salud Carlos III
- PI20/01063/Instituto de Salud Carlos III
- PI20/01063/Instituto de Salud Carlos III
- PI20/01063/Instituto de Salud Carlos III
- PI20/01063/Instituto de Salud Carlos III
- FPU19/03255/Ministerio de Ciencia, Innovación y Universidades
- PC060-061/Dirección General de Industria, Energia y Proyectos Estrategicos S3, Gobierno de Navarra
- PC060-061/Dirección General de Industria, Energia y Proyectos Estrategicos S3, Gobierno de Navarra
- PC060-061/Dirección General de Industria, Energia y Proyectos Estrategicos S3, Gobierno de Navarra
- PC060-061/Dirección General de Industria, Energia y Proyectos Estrategicos S3, Gobierno de Navarra
- PC060-061/Dirección General de Industria, Energia y Proyectos Estrategicos S3, Gobierno de Navarra
- FPU18/02244/Ministerio de Ciencia, Innovación y Universidades,Spain
- FPU21/01545/Ministerio de Ciencia, Innovación y Universidades,Spain
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

