Development of nanobodies targeting hepatocellular carcinoma and application of nanobody-based CAR-T technology
- PMID: 38610029
- PMCID: PMC11015683
- DOI: 10.1186/s12967-024-05159-x
Development of nanobodies targeting hepatocellular carcinoma and application of nanobody-based CAR-T technology
Abstract
Background: Chimeric antigen receptor T (CAR-T) cell therapy, as an emerging anti-tumor treatment, has garnered extensive attention in the study of targeted therapy of multiple tumor-associated antigens in hepatocellular carcinoma (HCC). However, the suppressive microenvironment and individual heterogeneity results in downregulation of these antigens in certain patients' cancer cells. Therefore, optimizing CAR-T cell therapy for HCC is imperative.
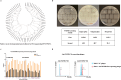
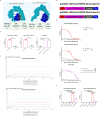
Methods: In this study, we administered FGFR4-ferritin (FGFR4-HPF) nanoparticles to the alpaca and constructed a phage library of nanobodies (Nbs) derived from alpaca, following which we screened for Nbs targeting FGFR4. Then, we conducted the functional validation of Nbs. Furthermore, we developed Nb-derived CAR-T cells and evaluated their anti-tumor ability against HCC through in vitro and in vivo validation.
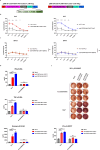
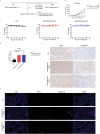
Results: Our findings demonstrated that we successfully obtained high specificity and high affinity Nbs targeting FGFR4 after screening. And the specificity of Nbs targeting FGFR4 was markedly superior to their binding to other members of the FGFR family proteins. Furthermore, the Nb-derived CAR-T cells, targeting FGFR4, exhibited significantly enhanced anti-tumor efficacy in both experiments when in vitro and in vivo.
Conclusions: In summary, the results of this study suggest that the CAR-T cells derived from high specificity and high affinity Nbs, targeting FGFR4, exhibited significantly enhanced anti-tumor efficacy in vitro and in vivo. This is an exploration of FGFR4 in the field of Nb-derived CAR-T cell therapy for HCC, holding promise for enhancing safety and effectiveness in the clinical treatment of HCC in the future.
Keywords: HCC; Nanobody; Nanoparticle; Nb-derived CAR-T cell therapy; Phage display.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
[Preparation of Trop2-Targeted CAR-T Cells Based on Nanobodies and Their Antitumor Effects Against Non-Small Cell Lung Cancer].Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Jan 20;56(1):198-205. doi: 10.12182/20250160107. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40109477 Free PMC article. Chinese.
-
Nanobody-derived bispecific CAR-T cell therapy enhances the anti-tumor efficacy of T cell lymphoma treatment.Mol Ther Oncolytics. 2023 Jul 28;30:86-102. doi: 10.1016/j.omto.2023.07.007. eCollection 2023 Sep 21. Mol Ther Oncolytics. 2023. PMID: 37593111 Free PMC article.
-
GPC3-IL7-CCL19-CAR-T primes immune microenvironment reconstitution for hepatocellular carcinoma therapy.Cell Biol Toxicol. 2023 Dec;39(6):3101-3119. doi: 10.1007/s10565-023-09821-w. Epub 2023 Oct 19. Cell Biol Toxicol. 2023. PMID: 37853185
-
Chimeric antigen receptor-engineered T-cell therapy for liver cancer.Hepatobiliary Pancreat Dis Int. 2018 Aug;17(4):301-309. doi: 10.1016/j.hbpd.2018.05.005. Epub 2018 May 24. Hepatobiliary Pancreat Dis Int. 2018. PMID: 29861325 Review.
-
The tumor microenvironment of hepatocellular carcinoma and its targeting strategy by CAR-T cell immunotherapy.Front Endocrinol (Lausanne). 2022 Aug 25;13:918869. doi: 10.3389/fendo.2022.918869. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36093115 Free PMC article. Review.
Cited by
-
Single domain antibody: Development and application in biotechnology and biopharma.Immunol Rev. 2024 Nov;328(1):98-112. doi: 10.1111/imr.13381. Epub 2024 Aug 21. Immunol Rev. 2024. PMID: 39166870 Free PMC article. Review.
-
Nanobody-enhanced chimeric antigen receptor T-cell therapy: overcoming barriers in solid tumors with VHH and VNAR-based constructs.Biomark Res. 2025 Mar 11;13(1):41. doi: 10.1186/s40364-025-00755-5. Biomark Res. 2025. PMID: 40069884 Free PMC article. Review.
-
Nanobodies targeting the tumor microenvironment and their formulation as nanomedicines.Mol Cancer. 2025 Mar 4;24(1):65. doi: 10.1186/s12943-025-02270-5. Mol Cancer. 2025. PMID: 40033293 Free PMC article. Review.
-
Application and prospect analysis of chimeric antigen receptor T-cell therapy in hepatocellular carcinoma treatment: a systematic review and meta-analysis.Front Immunol. 2025 Apr 7;16:1566976. doi: 10.3389/fimmu.2025.1566976. eCollection 2025. Front Immunol. 2025. PMID: 40260256 Free PMC article.
-
Nanomaterials Mediated Enhancement of CAR-T for HCC: Revolutionizing Immunotherapy Strategies.Int J Nanomedicine. 2025 Jun 13;20:7489-7500. doi: 10.2147/IJN.S527315. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40535838 Free PMC article. Review.
References
-
- Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2022YFC0870700/National Key R&D Program of Department of Science and Technology of China
- 92169201/Important Key Program of Natural Science Foundation of China (NSFC)
- 82150710553/Exchange Program of NSFC
- EKPG21-24/Emergency Key Program of Guangzhou National Laboratory
- 2022B1111020004/Key R&D Program of Department of Science and Technology of Guangdong
- 2021YFC2301904/National key research and development program
- SL2022A04J01923/Basic and Applied Basic Research Fund Committee of Guangdong Province
- 2023A1515010476/Guangdong Basic and Applied Basic Research Foundation
- 2023A04J2235/Guangzhou Science and technology plan project
- 82202036/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

