3D Kinematics Quantifies Gait Response to Levodopa earlier and to a more Comprehensive Extent than the MDS-Unified Parkinson's Disease Rating Scale in Patients with Motor Complications
- PMID: 38610081
- PMCID: PMC11233852
- DOI: 10.1002/mdc3.14016
3D Kinematics Quantifies Gait Response to Levodopa earlier and to a more Comprehensive Extent than the MDS-Unified Parkinson's Disease Rating Scale in Patients with Motor Complications
Abstract
Background: Quantitative 3D movement analysis using inertial measurement units (IMUs) allows for a more detailed characterization of motor patterns than clinical assessment alone. It is essential to discriminate between gait features that are responsive or unresponsive to current therapies to better understand the underlying pathophysiological basis and identify potential therapeutic strategies.
Objectives: This study aims to characterize the responsiveness and temporal evolution of different gait subcomponents in Parkinson's disease (PD) patients in their OFF and various ON states following levodopa administration, utilizing both wearable sensors and the gold-standard MDS-UPDRS motor part III.
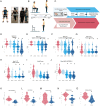
Methods: Seventeen PD patients were assessed while wearing a full-body set of 15 IMUs in their OFF state and at 20-minute intervals following the administration of a supra-threshold levodopa dose. Gait was reconstructed using a biomechanical model of the human body to quantify how each feature was modulated. Comparisons with non-PD control subjects were conducted in parallel.
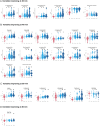
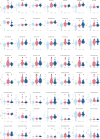
Results: Significant motor changes were observed in both the upper and lower limbs according to the MDS-UPDRS III, 40 minutes after levodopa intake. IMU-assisted 3D kinematics detected significant motor alterations as early as 20 minutes after levodopa administration, particularly in upper limbs metrics. Although all "pace-domain" gait features showed significant improvement in the Best-ON state, most rhythmicity, asymmetry, and variability features did not.
Conclusion: IMUs are capable of detecting motor alterations earlier and in a more comprehensive manner than the MDS-UPDRS III. The upper limbs respond more rapidly to levodopa, possibly reflecting distinct thresholds to levodopa across striatal regions.
Keywords: Parkinson's disease; gait; kinematic analysis; levodopa challenge test; wearable devices.
© 2024 International Parkinson and Movement Disorder Society.
Figures
Similar articles
-
Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa.J Neurol Sci. 2003 Aug 15;212(1-2):47-53. doi: 10.1016/s0022-510x(03)00104-7. J Neurol Sci. 2003. PMID: 12809998
-
Locomotor response to levodopa in fluctuating Parkinson's disease.Exp Brain Res. 2008 Feb;184(4):469-78. doi: 10.1007/s00221-007-1113-y. Epub 2007 Sep 8. Exp Brain Res. 2008. PMID: 17828529 Clinical Trial.
-
Effects of Continuous Dopaminergic Stimulation on Parkinson's Disease Gait: A Longitudinal Prospective Study with Levodopa Intestinal Gel Infusion.J Parkinsons Dis. 2024;14(4):843-853. doi: 10.3233/JPD-240003. J Parkinsons Dis. 2024. PMID: 38728203 Free PMC article.
-
Does levodopa slow or hasten the rate of progression of Parkinson's disease?J Neurol. 2005 Oct;252 Suppl 4:IV37-IV42. doi: 10.1007/s00415-005-4008-5. J Neurol. 2005. PMID: 16222436 Review.
-
Clinical predictors of freezing of gait in patients with Parkinson's disease: A systematic review.Clin Neurol Neurosurg. 2025 May;252:108848. doi: 10.1016/j.clineuro.2025.108848. Epub 2025 Mar 12. Clin Neurol Neurosurg. 2025. PMID: 40101322
Cited by
-
Systematic review of wearables assessing medication effect on motor function and symptoms in Parkinson's disease.NPJ Parkinsons Dis. 2025 May 22;11(1):135. doi: 10.1038/s41531-025-00943-y. NPJ Parkinsons Dis. 2025. PMID: 40404728 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

