Mapping cutaneous field carcinogenesis of nonmelanoma skin cancer using mesoscopic imaging of pro-inflammation cues
- PMID: 38610095
- PMCID: PMC11034840
- DOI: 10.1111/exd.15076
Mapping cutaneous field carcinogenesis of nonmelanoma skin cancer using mesoscopic imaging of pro-inflammation cues
Abstract
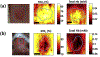
Nonmelanoma skin cancers remain the most widely diagnosed types of cancers globally. Thus, for optimal patient management, it has become imperative that we focus our efforts on the detection and monitoring of cutaneous field carcinogenesis. The concept of field cancerization (or field carcinogenesis), introduced by Slaughter in 1953 in the context of oral cancer, suggests that invasive cancer may emerge from a molecularly and genetically altered field affecting a substantial area of underlying tissue including the skin. A carcinogenic field alteration, present in precancerous tissue over a relatively large area, is not easily detected by routine visualization. Conventional dermoscopy and microscopy imaging are often limited in assessing the entire carcinogenic landscape. Recent efforts have suggested the use of noninvasive mesoscopic (between microscopic and macroscopic) optical imaging methods that can detect chronic inflammatory features to identify pre-cancerous and cancerous angiogenic changes in tissue microenvironments. This concise review covers major types of mesoscopic optical imaging modalities capable of assessing pro-inflammatory cues by quantifying blood haemoglobin parameters and hemodynamics. Importantly, these imaging modalities demonstrate the ability to detect angiogenesis and inflammation associated with actinically damaged skin. Representative experimental preclinical and human clinical studies using these imaging methods provide biological and clinical relevance to cutaneous field carcinogenesis in altered tissue microenvironments in the apparently normal epidermis and dermis. Overall, mesoscopic optical imaging modalities assessing chronic inflammatory hyperemia can enhance the understanding of cutaneous field carcinogenesis, offer a window of intervention and monitoring for actinic keratoses and nonmelanoma skin cancers and maximise currently available treatment options.
Keywords: hyperemia; inflammation; optical imaging; skin neoplasms.
© 2024 The Authors. Experimental Dermatology published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no conflict of interests.
Figures

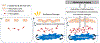
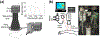


Similar articles
-
Variation within and between digital pathology and light microscopy for the diagnosis of histopathology slides: blinded crossover comparison study.Health Technol Assess. 2025 Jul;29(30):1-75. doi: 10.3310/SPLK4325. Health Technol Assess. 2025. PMID: 40654002 Free PMC article.
-
Interventions for melanoma in situ, including lentigo maligna.Cochrane Database Syst Rev. 2014 Dec 19;2014(12):CD010308. doi: 10.1002/14651858.CD010308.pub2. Cochrane Database Syst Rev. 2014. PMID: 25526608 Free PMC article.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Machine reading and recovery of colors for hemoglobin-related bioassays and bioimaging.Sci Adv. 2025 Jun 6;11(23):eadt4831. doi: 10.1126/sciadv.adt4831. Epub 2025 Jun 4. Sci Adv. 2025. PMID: 40465724 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

