The Net Clinical Outcome of Dual-Pathway Inhibition in Clinical Practice: The "Xarelto plus Acetylsalicylic Acid: Treatment Patterns and Outcomes in Patients with Atherosclerosis" Registry
- PMID: 38610724
- PMCID: PMC11012443
- DOI: 10.3390/jcm13071956
The Net Clinical Outcome of Dual-Pathway Inhibition in Clinical Practice: The "Xarelto plus Acetylsalicylic Acid: Treatment Patterns and Outcomes in Patients with Atherosclerosis" Registry
Abstract
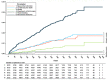
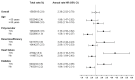
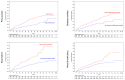
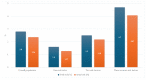
Background: In the COMPASS trial, the combination of acetylsalicylic acid (ASA) plus 2.5 mg rivaroxaban twice daily (dual-pathway inhibition, DPI) has been shown to be superior to ASA monotherapy for the reduction in ischemic major adverse cardiovascular events (MACEs, i.e., cardiovascular death, stroke, or myocardial infarction). Methods: The international XATOA registry (Xarelto plus Acetylsalicylic acid: Treatment patterns and Outcomes in patients with Atherosclerosis) is a prospective post-approval registry that investigates the cardiovascular outcomes of patients taking ASA plus 2.5 mg rivaroxaban. The aim of this pre-specified analysis was to determine the net clinical outcome (NCO), i.e., a combination of MACEs and bleeding events, of DPI in patients from daily clinical practice. Results: Among the 5615 patients, the presence of multiple risk factors resulted in an increase in the total risk of experiencing an NCO event, e.g., from 1.27% (one risk factor) to 2.18% (two risk factors) and 4.07% (three or more risk factors), respectively, with ischemic MACE representing the primary driver of bleeding complications. Conclusions: In the real-world XATOA registry, the annual rate of NCO events was low and numerically similar to those seen in the treatment group in the randomized COMPASS trial.
Keywords: XATOA registry; dual-pathway inhibition; net clinical benefit.
Conflict of interest statement
A.B. has received consultant and/or speaker fees from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cook Medical, Daiichi Sankyo, Medtronic, Pfizer, and Spectranetics/Philips. K.A.A.F. has received honoraria from Bayer, AstraZeneca, and Milestone. A.G. and K.V. are employees of Bayer AG, Germany. J.S. has received consultant and/or speaker fees from Abbott, Alexion, AstraZeneca, Bayer, Berlin-Chemie, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, BMS, Daiichi Sankyo, Medscape, Medtronic, Menarini, Organon, Pfizer, Saja, Servier, and WebMD. He reports ownership of Swiss EP and CorXL. J.S. has received grant support through his previous institution (University Hospital Zurich) from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic.
Figures
References
-
- Task Force M., Montalescot G., Sechtem U., Achenbach S., Andreotti F., Arden C., Budaj A., Bugiardini R., Crea F., Cuisset T., et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. - DOI - PubMed
-
- CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. - PubMed
-
- Bonaca M.P., Scirica B.M., Creager M.A., Olin J., Bounameaux H., Dellborg M., Lamp J.M., Murphy S.A., Braunwald E., Morrow D.A. Vorapaxar in patients with peripheral artery disease: Results from TRA2degreesP-TIMI 50. Circulation. 2013;127:1522–1529. doi: 10.1161/CIRCULATIONAHA.112.000679. - DOI - PubMed
-
- Windecker S., Kolh P., Alfonso F., Collet J.P., Cremer J., Falk V., Filippatos G., Hamm C., Head S.J., Juni P., et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

