Targeting Siglec-Sialylated MUC1 Immune Axis in Cancer
- PMID: 38611013
- PMCID: PMC11011055
- DOI: 10.3390/cancers16071334
Targeting Siglec-Sialylated MUC1 Immune Axis in Cancer
Abstract
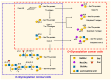
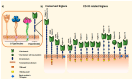
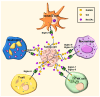
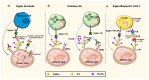
Siglecs play a key role in mediating cell-cell interactions via the recognition of different sialylated glycoconjugates, including tumor-associated MUC1, which can lead to the activation or inhibition of the immune response. The activation occurs through the signaling of Siglecs with the cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM)-containing proteins, while the inhibition signal is a result of the interaction of intracellular immunoreceptor tyrosine-based inhibition motif (ITIM)-bearing receptors. The interaction of tumor-associated MUC1 sialylated glycans with Siglecs via ITIM motifs decreases antitumor immunity. Consequently, these interactions are expected to play a key role in tumor evasion. Efforts to modulate the response of immune cells by blocking the immune-suppressive effects of inhibitory Siglecs, driving immune-activating Siglecs, and/or altering the synthesis and expression of the sialic acid glycocalyx are new therapeutic strategies deserving further investigation. We will highlight the role of Siglec's family receptors in immune evasion through interactions with glycan ligands in their natural context, presented on the protein such as MUC1, factors affecting their fine binding specificities, such as the role of multivalency either at the ligand or receptor side, their spatial organization, and finally the current and future therapeutic interventions targeting the Siglec-sialylated MUC1 immune axis in cancer.
Keywords: MUC1; Siglecs; glycocode; immune evasion; sialoglycans.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Tools to study and target the Siglec-sialic acid axis in cancer.FEBS J. 2021 Nov;288(21):6206-6225. doi: 10.1111/febs.15647. Epub 2020 Dec 21. FEBS J. 2021. PMID: 33251699 Review.
-
Siglec Signaling in the Tumor Microenvironment.Front Immunol. 2021 Dec 13;12:790317. doi: 10.3389/fimmu.2021.790317. eCollection 2021. Front Immunol. 2021. PMID: 34966391 Free PMC article. Review.
-
Sensing the neuronal glycocalyx by glial sialic acid binding immunoglobulin-like lectins.Neuroscience. 2014 Sep 5;275:113-24. doi: 10.1016/j.neuroscience.2014.05.061. Epub 2014 Jun 9. Neuroscience. 2014. PMID: 24924144 Review.
-
New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins.J Biol Chem. 2000 Mar 24;275(12):8625-32. doi: 10.1074/jbc.275.12.8625. J Biol Chem. 2000. PMID: 10722702
-
Non-canonical roles of Siglecs: Beyond sialic acid-binding and immune cell modulation.Mol Aspects Med. 2023 Apr;90:101145. doi: 10.1016/j.mam.2022.101145. Epub 2022 Sep 22. Mol Aspects Med. 2023. PMID: 36153172 Review.
Cited by
-
Synthesis and Thermodynamic Evaluation of Sialyl-Tn MUC1 Glycopeptides Binding to Macrophage Galactose-Type Lectin.Chembiochem. 2024 Sep 16;25(18):e202400391. doi: 10.1002/cbic.202400391. Epub 2024 Jul 30. Chembiochem. 2024. PMID: 38877657
-
Implications of Mucin-Type O-Glycosylation in Alzheimer's Disease.Molecules. 2025 Apr 24;30(9):1895. doi: 10.3390/molecules30091895. Molecules. 2025. PMID: 40363702 Free PMC article. Review.
-
Comprehensive analysis of α2,3-sialyltransferases as prognostic biomarkers and immunotherapy targets in kidney renal clear cell carcinoma.Cancer Cell Int. 2025 Feb 7;25(1):36. doi: 10.1186/s12935-025-03640-1. Cancer Cell Int. 2025. PMID: 39920744 Free PMC article.
-
Cancer stem cells in personalized therapy: mechanisms, microenvironment crosstalk, and therapeutic vulnerabilities.Front Cell Dev Biol. 2025 Jul 30;13:1619597. doi: 10.3389/fcell.2025.1619597. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40809695 Free PMC article. Review.
-
Mechanistic and Therapeutic Implications of Protein and Lipid Sialylation in Human Diseases.Int J Mol Sci. 2024 Nov 7;25(22):11962. doi: 10.3390/ijms252211962. Int J Mol Sci. 2024. PMID: 39596031 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

