Immune-Related Adverse Events Due to Cancer Immunotherapy: Immune Mechanisms and Clinical Manifestations
- PMID: 38611115
- PMCID: PMC11011060
- DOI: 10.3390/cancers16071440
Immune-Related Adverse Events Due to Cancer Immunotherapy: Immune Mechanisms and Clinical Manifestations
Abstract
The landscape of cancer treatment has undergone a significant transformation with the introduction of Immune Checkpoint Inhibitors (ICIs). Patients undergoing these treatments often report prolonged clinical and radiological responses, albeit with a potential risk of developing immune-related adverse events (irAEs). Here, we reviewed and discussed the mechanisms of action of ICIs and their pivotal role in regulating the immune system to enhance the anti-tumor immune response. We scrutinized the intricate pathogenic mechanisms responsible for irAEs, arising from the evasion of self-tolerance checkpoints due to drug-induced immune modulation. We also summarized the main clinical manifestations due to irAEs categorized by organ types, detailing their incidence and associated risk factors. The occurrence of irAEs is more frequent when ICIs are combined; with neurological, cardiovascular, hematological, and rheumatic irAEs more commonly linked to PD1/PD-L1 inhibitors and cutaneous and gastrointestinal irAEs more prevalent with CTLA4 inhibitors. Due to the often-nonspecific signs and symptoms, the diagnosis of irAEs (especially for those rare ones) can be challenging. The differential with primary autoimmune disorders becomes sometimes intricate, given the clinical and pathophysiological similarities. In conclusion, considering the escalating use of ICIs, this area of research necessitates additional clinical studies and practical insights, especially the development of biomarkers for predicting immune toxicities. In addition, there is a need for heightened education for both clinicians and patients to enhance understanding and awareness.
Keywords: ICI; anti-CTLA4; anti-PD1/PD-L1; immune checkpoint inhibitors; immune-related adverse events; irAEs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
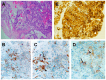

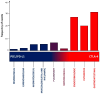
References
-
- Lebbé C., Weber J.S., Maio M., Neyns B., Harmankaya K., Hamid O., O’Day S.J., Konto C., Cykowski L., McHenry M.B., et al. Survival Follow-up and Ipilimumab Retreatment of Patients with Advanced Melanoma Who Received Ipilimumab in Prior Phase II Studies. Ann. Oncol. 2014;25:2277–2284. doi: 10.1093/annonc/mdu441. - DOI - PMC - PubMed
-
- Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.-J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F., et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. - DOI - PMC - PubMed
-
- Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Sosman J.A., Atkins M.B., Leming P.D., et al. Five-Year Survival and Correlates among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non–Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncol. 2019;5:1411–1420. doi: 10.1001/jamaoncol.2019.2187. - DOI - PMC - PubMed
-
- Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

