Simultaneous Formation of Polyhydroxyurethanes and Multicomponent Semi-IPN Hydrogels
- PMID: 38611138
- PMCID: PMC11013152
- DOI: 10.3390/polym16070880
Simultaneous Formation of Polyhydroxyurethanes and Multicomponent Semi-IPN Hydrogels
Abstract
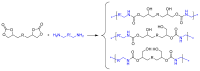
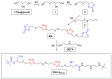
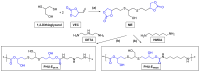
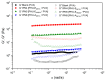
This study introduces an efficient strategy for synthesizing polyhydroxyurethane-based multicomponent hydrogels with enhanced rheological properties. In a single-step process, 3D materials composed of Polymer 1 (PHU) and Polymer 2 (PVA or gelatin) were produced. Polymer 1, a crosslinked polyhydroxyurethane (PHU), grew within a colloidal solution of Polymer 2, forming an interconnected network. The synthesis of Polymer 1 utilized a Non-Isocyanate Polyurethane (NIPU) methodology based on the aminolysis of bis(cyclic carbonate) (bisCC) monomers derived from 1-thioglycerol and 1,2-dithioglycerol (monomers A and E, respectively). This method, applied for the first time in Semi-Interpenetrating Network (SIPN) formation, demonstrated exceptional orthogonality since the functional groups in Polymer 2 do not interfere with Polymer 1 formation. Optimizing PHU formation involved a 20-trial methodology, identifying influential variables such as polymer concentration, temperature, solvent (an aprotic and a protic solvent), and the organo-catalyst used [a thiourea derivative (TU) and 1,8-diazabicyclo [5.4.0]undec-7-ene (DBU)]. The highest molecular weights were achieved under near-bulk polymerization conditions using TU-protic and DBU-aprotic as catalyst-solvent combinations. Monomer E-based PHU exhibited higher Mw¯ than monomer A-based PHU (34.1 kDa and 16.4 kDa, respectively). Applying the enhanced methodology to prepare 10 multicomponent hydrogels using PVA or gelatin as the polymer scaffold revealed superior rheological properties in PVA-based hydrogels, exhibiting solid-like gel behavior. Incorporating monomer E enhanced mechanical properties and elasticity (with loss tangent values of 0.09 and 0.14). SEM images unveiled distinct microstructures, including a sponge-like pattern in certain PVA-based hydrogels when monomer A was chosen, indicating the formation of highly superporous interpenetrated materials. In summary, this innovative approach presents a versatile methodology for obtaining advanced hydrogel-based systems with potential applications in various biomedical fields.
Keywords: IPN; NIPU; PHU; SIPN; cyclic carbonates; functional polymers; interpenetrated networks; porous materials; rheological properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

