Investigation of Silk Fibroin/Poly(Acrylic Acid) Interactions in Aqueous Solution
- PMID: 38611194
- PMCID: PMC11013473
- DOI: 10.3390/polym16070936
Investigation of Silk Fibroin/Poly(Acrylic Acid) Interactions in Aqueous Solution
Abstract
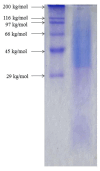
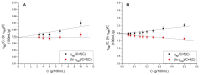
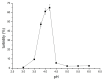
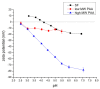
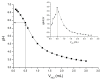
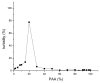
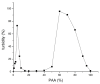
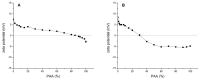
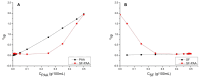
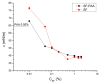
Silk fibroin (SF) is a protein with many outstanding properties (superior biocompatibility, mechanical strength, etc.) and is often used in many advanced applications (epidermal sensors, tissue engineering, etc.). The properties of SF-based biomaterials may additionally be tuned by SF interactions with other (bio)polymers. Being a weak amphoteric polyelectrolyte, SF may form polyelectrolyte complexes (PECs) with other polyelectrolytes of opposite charge, such as poly(acrylic acid) (PAA). PAA is a widely used, biocompatible, synthetic polyanion. Here, we investigate PEC formation between SF and PAA of two different molecular weights (MWs), low and high, using various techniques (turbidimetry, zeta potential measurements, capillary viscometry, and tensiometry). The colloidal properties of SF isolated from Bombyx mori and of PAAs (MW, overlap concentration, the influence of pH on zeta potential, adsorption at air/water interface) were determined to identify conditions for the SF-PAA electrostatic interaction. It was shown that SF-PAA PEC formation takes place at different SF:PAA ratios, at pH 3, for both high and low MW PAA. SF-PAA PEC's properties (phase separation, charge, and surface activity) are influenced by the SF:PAA mass ratio and/or the MW of PAA. The findings on the interactions contribute to the future development of SP-PAA PEC-based films and bioadhesives with tailored properties.
Keywords: complex coacervation; poly(acrylic acid); polyelectrolyte complex (PEC); polymer/polymer interaction; silk fibroin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Allardyce B.J., Rajkhowa R., Dilley R.J., Atlas M.D., Kaur J., Wang X. The impact of degumming conditions on the properties of silk films for biomedical applications. Text. Res. J. 2016;86:275–287. doi: 10.1177/0040517515586166. - DOI
Grants and funding
- 14869304/Ministry of Science and Higher Education of the Republic of Kazakhstan
- 451-03-66/2024-03/200134/Ministry of Science, Technological Development and Innovation of the Republic of Serbia
- 451-03-65/2024-03/200134/Ministry of Science, Technological Development and Innovation of the Republic of Serbia
LinkOut - more resources
Full Text Sources

