Comments and Illustrations of Ultrasound Findings in Extrapulmonary Tuberculosis Manifestations
- PMID: 38611619
- PMCID: PMC11011484
- DOI: 10.3390/diagnostics14070706
Comments and Illustrations of Ultrasound Findings in Extrapulmonary Tuberculosis Manifestations
Abstract
This review describes the appearance of extrapulmonary tuberculosis manifestations in comprehensive and multiparametric ultrasound imaging. The aim is to increase awareness of typical ultrasound findings regarding extrapulmonary tuberculosis, correlate those with pathological features, and facilitate differential diagnosis. Point of care ultrasound protocols can be used as a screening method in high-risk populations, although the negative findings do not exclude tuberculosis. Conversely, the diagnosis of extrapulmonary tuberculosis can never be made using ultrasound alone, as many ultrasound findings in extrapulmonary tuberculosis are non-specific. However, ultrasound-based sampling techniques can significantly facilitate the collection of samples for microbiological or molecular proof of tuberculosis, as well as facilitating the establishment of alternative diagnoses.
Keywords: contrast enhanced ultrasonography; endoscopic ultrasound; extrapulmonary manifestations; tuberculosis; ultrasonography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
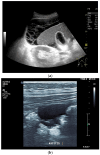


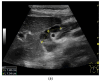




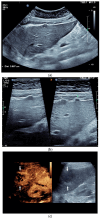


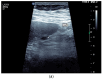










References
-
- WHO Global Tuberculosis Report 2023. [(accessed on 7 November 2023)]. Available online: www.who.int/tb.
Publication types
LinkOut - more resources
Full Text Sources

