Electrochemical Monitoring in Anticoagulation Therapy
- PMID: 38611733
- PMCID: PMC11012951
- DOI: 10.3390/molecules29071453
Electrochemical Monitoring in Anticoagulation Therapy
Abstract
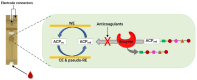
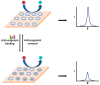
The process of blood coagulation, wherein circulating blood transforms into a clot in response to an internal or external injury, is a critical physiological mechanism. Monitoring this coagulation process is vital to ensure that blood clotting neither occurs too rapidly nor too slowly. Anticoagulants, a category of medications designed to prevent and treat blood clots, require meticulous monitoring to optimise dosage, enhance clinical outcomes, and minimise adverse effects. This review article delves into the various stages of blood coagulation, explores commonly used anticoagulants and their targets within the coagulation enzyme system, and emphasises the electrochemical methods employed in anticoagulant testing. Electrochemical sensors for anticoagulant monitoring are categorised into two types. The first type focuses on assays measuring thrombin activity via electrochemical techniques. The second type involves modified electrode surfaces that either directly measure the redox behaviours of anticoagulants or monitor the responses of standard redox probes in the presence of these drugs. This review comprehensively lists different electrode compositions and their detection and quantification limits. Additionally, it discusses the potential of employing a universal calibration plot to replace individual drug-specific calibrations. The presented insights are anticipated to significantly contribute to the sensor community's efforts in this field.
Keywords: anticoagulants; coagulation; electrochemical sensors; factor Xa; point of care; thrombin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

