Medium-Sized Ring Expansion Strategies: Enhancing Small-Molecule Library Development
- PMID: 38611841
- PMCID: PMC11013129
- DOI: 10.3390/molecules29071562
Medium-Sized Ring Expansion Strategies: Enhancing Small-Molecule Library Development
Abstract
The construction of a small molecule library that includes compounds with medium-sized rings is increasingly essential in drug discovery. These compounds are essential for identifying novel therapeutic agents capable of targeting "undruggable" targets through high-throughput and high-content screening, given their structural complexity and diversity. However, synthesizing medium-sized rings presents notable challenges, particularly with direct cyclization methods, due to issues such as transannular strain and reduced degrees of freedom. This review presents an overview of current strategies in synthesizing medium-sized rings, emphasizing innovative approaches like ring-expansion reactions. It highlights the challenges of synthesis and the potential of these compounds to diversify the chemical space for drug discovery, underscoring the importance of medium-sized rings in developing new bioactive compounds.
Keywords: bioactive compound discovery; diversity-oriented synthesis; medium-sized ring synthesis; natural product mimics; ring-expansion reactions.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



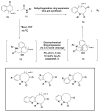


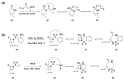
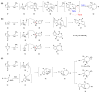

References
-
- Barba-Ostria C., Carrera-Pacheco S.E., Gonzalez-Pastor R., Heredia-Moya J., Mayorga-Ramos A., Rodríguez-Pólit C., Zúñiga-Miranda J., Arias-Almeida B., Guamán L.P. Evaluation of biological activity of natural compounds: Current trends and methods. Molecules. 2022;27:4490. doi: 10.3390/molecules27144490. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

