Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective
- PMID: 38611908
- PMCID: PMC11013693
- DOI: 10.3390/molecules29071629
Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective
Abstract
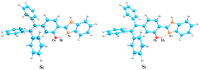
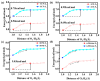
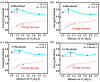
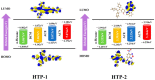
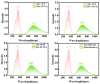
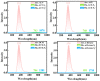
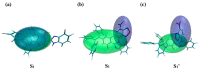
As reversible fluorescent probes, HTP-1 and HTP-2 have favourable applications for the detection of Zn2+ and H2S. Herein, the impact of solvent on the excited-state intramolecular proton transfer (ESIPT) of HTP-1 and HTP-2 was comprehensively investigated. The obtained geometric parameters and infrared (IR) vibrational analysis associated with the intramolecular hydrogen bond (IHB) indicated that the strength of IHB for HTP-1 was weakened in the excited state. Moreover, structural torsion and almost no ICT behaviour indicated that the ESIPT process did not occur in HTP-1. Nevertheless, when the 7-nitro-1,2,3-benzoxadiazole (NBD) group replaced the H atom, the IHB strength of HTP-2 was enhanced after photoexcitation, which inhibited the twisting of tetraphenylethylene, thereby opening the ESIPT channel. Notably, hole-electron analysis and frontier molecular orbitals revealed that the charge decoupling effect was the reason for the fluorescence quenching of HTP-2. Furthermore, the potential energy curves (PECs) revealed that HTP-2 was more inclined to the ESIPT process in polar solvents than in nonpolar solvents. With a decrease in solvent polarity, it was more conducive to the ESIPT process. Our study systematically presents the ESIPT process and different detection mechanisms of the two reversible probe molecules regulated by solvent polarity, providing new insights into the design and development of novel fluorescent probes.
Keywords: excited-state intramolecular proton transfer; intramolecular charge transfer; intramolecular hydrogen bond.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

