Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA)
- PMID: 38612386
- PMCID: PMC11012217
- DOI: 10.3390/ijms25073574
Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA)
Abstract
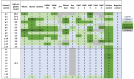
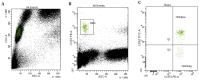
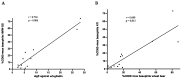
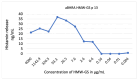
Wheat allergy dependent on augmentation factors (WALDA) is the most common gluten allergy in adults. IgE-mediated sensitizations are directed towards ω5-gliadin but also to other wheat allergens. The value of the different in vitro cellular tests, namely the basophil activation test (BAT) and the active (aBHRA) and passive basophil histamine-release assays (pBHRA), in the detection of sensitization profiles beyond ω5-gliadin has not been compared. Therefore, 13 patients with challenge-confirmed, ω5-gliadin-positive WALDA and 11 healthy controls were enrolled. Specific IgE (sIgE), skin prick tests, BATs, aBHRA, and pBHRA were performed with allergen test solutions derived from wheat and other cereals, and results were analyzed and compared. This study reveals a distinct and highly individual reactivity of ω5-gliadin-positive WALDA patients to a range of wheat allergens beyond ω5-gliadin in cellular in vitro tests and SPT. In the BAT, for all tested allergens (gluten, high-molecular-weight glutenin subunits, α-amylase/trypsin inhibitors (ATIs), alcohol-free wheat beer, hydrolyzed wheat proteins (HWPs), rye gluten and secalins), basophil activation in patients was significantly higher than in controls (p = 0.004-p < 0.001). Similarly, significant histamine release was detected in the aBHRA for all test substances, exceeding the cut-off of 10 ng/mL in all tested allergens in 50% of patients. The dependency of tests on sIgE levels against ω5-gliadin differed; in the pBHRA, histamine release to any test substances could only be detected in patients with sIgE against ω5-gliadin ≥ 7.7 kU/L, whereas aBHRA also showed high reactivity in less sensitized patients. In most patients, reactivity to HWPs, ATIs, and rye allergens was observed. Additionally, alcohol-free wheat beer was first described as a promising test substance in ω5-gliadin-positive WALDA. Thus, BAT and aBHRA are valuable tools for the identification of sensitization profiles in WALDA.
Keywords: IgE; WALDA; WDEIA; basophil activation test; basophil histamine-release assay; food allergy; gluten; hydrolyzed wheat proteins; rye; wheat allergy dependent on augmentation factors.
Conflict of interest statement
P.S.S. is acting as scientific advisor for RefLab ApS. B.E. reports nonfinancial support from Bühlmann Laboratories outside the submitted work, and K.B. reports honoraria from Thermofisher for oral presentations. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Kraft M., Dolle-Bierke S., Renaudin J.M., Rueff F., Hofmeier K.S., Treudler R., Pfohler C., Hawranek T., Poziomkowska-Gesicka I., Jappe U., et al. Wheat Anaphylaxis in Adults Differs from Reactions to Other Types of Food. J. Allergy Clin. Immunol. Pract. 2021;9:2844–2852.e5. doi: 10.1016/j.jaip.2021.03.037. - DOI - PubMed
-
- Dolle-Bierke S., Hofer V., Francuzik W., Naher A.F., Bilo M.B., Cichocka-Jarosz E., Lopes de Oliveira L.C., Fernandez-Rivas M., Garcia B.E., Hartmann K., et al. Food-Induced Anaphylaxis: Data from the European Anaphylaxis Registry. J. Allergy Clin. Immunol. Pract. 2023;11:2069–2079.e7. doi: 10.1016/j.jaip.2023.03.026. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

