Co-Culture of P. gingivalis and F. nucleatum Synergistically Elevates IL-6 Expression via TLR4 Signaling in Oral Keratinocytes
- PMID: 38612423
- PMCID: PMC11011619
- DOI: 10.3390/ijms25073611
Co-Culture of P. gingivalis and F. nucleatum Synergistically Elevates IL-6 Expression via TLR4 Signaling in Oral Keratinocytes
Abstract
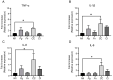
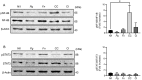
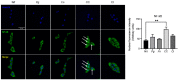
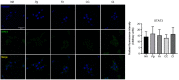
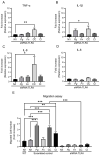
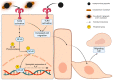
Periodontitis, characterized by persistent inflammation in the periodontium, is intricately connected to systemic diseases, including oral cancer. Bacteria, such as Porphyromonas gingivalis and Fusobacterium nucleatum, play a pivotal role in periodontitis development because they contribute to dysbiosis and tissue destruction. Thus, comprehending the interplay between these bacteria and their impacts on inflammation holds significant relevance in clinical understanding and treatment advancement. In the present work, we explored, for the first time, their impacts on the expressions of pro-inflammatory mediators after infecting oral keratinocytes (OKs) with a co-culture of pre-incubated P. gingivalis and F. nucleatum. Our results show that the co-culture increases IL-1β, IL-8, and TNF-α expressions, synergistically augments IL-6, and translocates NF-kB to the cell nucleus. These changes in pro-inflammatory mediators-associated with chronic inflammation and cancer-correlate with an increase in cell migration following infection with the co-cultured bacteria or P. gingivalis alone. This effect depends on TLR4 because TLR4 knockdown notably impacts IL-6 expression and cell migration. Our study unveils, for the first time, crucial insights into the outcomes of their co-culture on virulence, unraveling the role of bacterial interactions in polymicrobial diseases and potential links to oral cancer.
Keywords: Fusobacterium nucleatum; Porphyromonas gingivalis; co-culture; cytokines; inflammation; oral cancer; periodontitis; toll-like receptor 4.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

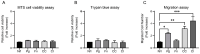
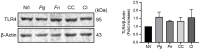
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

