Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist's Key Role
- PMID: 38612431
- PMCID: PMC11011777
- DOI: 10.3390/ijms25073618
Multidisciplinary Approach to the Diagnosis of Idiopathic Interstitial Pneumonias: Focus on the Pathologist's Key Role
Abstract
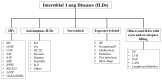
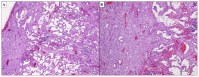
Idiopathic Interstitial Pneumonias (IIPs) are a heterogeneous group of the broader category of Interstitial Lung Diseases (ILDs), pathologically characterized by the distortion of lung parenchyma by interstitial inflammation and/or fibrosis. The American Thoracic Society (ATS)/European Respiratory Society (ERS) international multidisciplinary consensus classification of the IIPs was published in 2002 and then updated in 2013, with the authors emphasizing the need for a multidisciplinary approach to the diagnosis of IIPs. The histological evaluation of IIPs is challenging, and different types of IIPs are classically associated with specific histopathological patterns. However, morphological overlaps can be observed, and the same histopathological features can be seen in totally different clinical settings. Therefore, the pathologist's aim is to recognize the pathologic-morphologic pattern of disease in this clinical setting, and only after multi-disciplinary evaluation, if there is concordance between clinical and radiological findings, a definitive diagnosis of specific IIP can be established, allowing the optimal clinical-therapeutic management of the patient.
Keywords: desquamative interstitial pneumonia; diffuse alveolar damage; idiopathic interstitial pneumonias; idiopathic pulmonary fibrosis; interstitial lung disease; multidisciplinary discussion; nonspecific interstitial pneumonia; organizing pneumonia; pleuroparenchymal fibroelastosis; usual interstitial pneumonia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Lucà S., Zannini G., Morgillo F., Della Corte C.M., Fiorelli A., Zito Marino F., Campione S., Vicidomini G., Guggino G., Ronchi A., et al. The prognostic value of histopathology in invasive lung adenocarcinoma: A comparative review of the main proposed grading systems. Expert. Rev. Anticancer Ther. 2023;23:265–277. doi: 10.1080/14737140.2023.2179990. - DOI - PubMed
-
- Lucà S., Franco R., Napolitano A., Soria V., Ronchi A., Zito Marino F., Della Corte C.M., Morgillo F., Fiorelli A., Luciano A., et al. PATZ1 in Non-Small Cell Lung Cancer: A New Biomarker That Negatively Correlates with PD-L1 Expression and Suppresses the Malignant Phenotype. Cancers. 2023;15:2190. doi: 10.3390/cancers15072190. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

