Molecular Mechanisms of Neuroprotection after the Intermittent Exposures of Hypercapnic Hypoxia
- PMID: 38612476
- PMCID: PMC11011936
- DOI: 10.3390/ijms25073665
Molecular Mechanisms of Neuroprotection after the Intermittent Exposures of Hypercapnic Hypoxia
Abstract
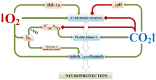
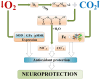
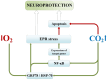
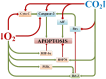
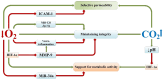
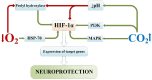
The review introduces the stages of formation and experimental confirmation of the hypothesis regarding the mutual potentiation of neuroprotective effects of hypoxia and hypercapnia during their combined influence (hypercapnic hypoxia). The main focus is on the mechanisms and signaling pathways involved in the formation of ischemic tolerance in the brain during intermittent hypercapnic hypoxia. Importantly, the combined effect of hypoxia and hypercapnia exerts a more pronounced neuroprotective effect compared to their separate application. Some signaling systems are associated with the predominance of the hypoxic stimulus (HIF-1α, A1 receptors), while others (NF-κB, antioxidant activity, inhibition of apoptosis, maintenance of selective blood-brain barrier permeability) are mainly modulated by hypercapnia. Most of the molecular and cellular mechanisms involved in the formation of brain tolerance to ischemia are due to the contribution of both excess carbon dioxide and oxygen deficiency (ATP-dependent potassium channels, chaperones, endoplasmic reticulum stress, mitochondrial metabolism reprogramming). Overall, experimental studies indicate the dominance of hypercapnia in the neuroprotective effect of its combined action with hypoxia. Recent clinical studies have demonstrated the effectiveness of hypercapnic-hypoxic training in the treatment of childhood cerebral palsy and diabetic polyneuropathy in children. Combining hypercapnic hypoxia with pharmacological modulators of neuro/cardio/cytoprotection signaling pathways is likely to be promising for translating experimental research into clinical medicine.
Keywords: A1 adenosine receptors; HIF-1α; antioxidant systems; apoptosis inhibition; blood–brain barrier permeability; chaperones; endoplasmic reticulum; hypercapnia; hypoxia; mitochondrial ATP-dependent potassium channels; neuroprotection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

