FL118 Is a Potent Therapeutic Agent against Chronic Myeloid Leukemia Resistant to BCR-ABL Inhibitors through Targeting RNA Helicase DDX5
- PMID: 38612503
- PMCID: PMC11011477
- DOI: 10.3390/ijms25073693
FL118 Is a Potent Therapeutic Agent against Chronic Myeloid Leukemia Resistant to BCR-ABL Inhibitors through Targeting RNA Helicase DDX5
Abstract
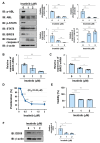
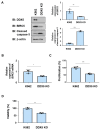
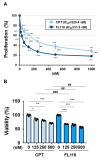
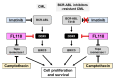
Chronic myeloid leukemia (CML) is induced by the expression of the fused tyrosine kinase BCR-ABL, which is caused by a chromosomal translocation. BCR-ABL inhibitors have been used to treat CML; however, the acquisition of resistance by CML cells during treatment is a serious issue. We herein demonstrated that BCR-ABL induced the expression of the RNA helicase DDX5 in K562 cells derived from CML patients in a manner that was dependent on its kinase activity, which resulted in cell proliferation and survival. The knockout of DDX5 decreased the expression of BIRC5 (survivin) and activated caspase 3, leading to apoptosis in K562 cells. Similar results were obtained in cells treated with FL118, an inhibitor of DDX5 and a derivative compound of camptothecin (CPT). Furthermore, FL118 potently induced apoptosis not only in Ba/F3 cells expressing BCR-ABL, but also in those expressing the BCR-ABL T315I mutant, which is resistant to BCR-ABL inhibitors. Collectively, these results revealed that DDX5 is a critical therapeutic target in CML and that FL118 is an effective candidate compound for the treatment of BCR-ABL inhibitor-resistant CML.
Keywords: BCR-ABL; DDX5; FL118; apoptosis; chronic myeloid leukemia (CML).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Novel Mechanism by a Bis-Pyridinium Fullerene Derivative to Induce Apoptosis by Enhancing the MEK-ERK Pathway in a Reactive Oxygen Species-Independent Manner in BCR-ABL-Positive Chronic Myeloid Leukemia-Derived K562 Cells.Int J Mol Sci. 2022 Jan 11;23(2):749. doi: 10.3390/ijms23020749. Int J Mol Sci. 2022. PMID: 35054935 Free PMC article.
-
Discovery of a highly potent kinase inhibitor capable of overcoming multiple imatinib-resistant ABL mutants for chronic myeloid leukemia (CML).Eur J Pharmacol. 2021 Apr 15;897:173944. doi: 10.1016/j.ejphar.2021.173944. Epub 2021 Feb 11. Eur J Pharmacol. 2021. PMID: 33581133
-
Curcumin derivative C817 inhibits proliferation of imatinib-resistant chronic myeloid leukemia cells with wild-type or mutant Bcr-Abl in vitro.Acta Pharmacol Sin. 2014 Mar;35(3):401-9. doi: 10.1038/aps.2013.180. Epub 2014 Feb 3. Acta Pharmacol Sin. 2014. PMID: 24487968 Free PMC article.
-
Characterization of cancer stem cells in chronic myeloid leukaemia.Biochem Soc Trans. 2007 Nov;35(Pt 5):1347-51. doi: 10.1042/BST0351347. Biochem Soc Trans. 2007. PMID: 17956348 Review.
-
[Recent Advance of Newly Therapy for Chronic Myeloid Leukemia with BCR-ABLT315I Mutation--Review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2023 Oct;31(5):1579-1583. doi: 10.19746/j.cnki.issn.1009-2137.2023.05.052. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2023. PMID: 37846720 Review. Chinese.
References
-
- de Klein A., van Kessel A.G., Grosveld G., Bartram C.R., Hagemeijer A., Bootsma D., Spurr N.K., Heisterkamp N., Groffen J., Stephenson J.R. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. - DOI - PubMed
-
- Zhao X., Ghaffari S., Lodish H., Malashkevich V.N., Kim P.S. Structure of the Bcr-Abl oncoprotein oligomerization domain. Nat. Struct. Biol. 2002;9:117–120. - PubMed
-
- Sonoyama J., Matsumura I., Ezoe S., Satoh Y., Zhang X., Kataoka Y., Takai E., Mizuki M., Machii T., Wakao H., et al. Functional cooperation among Ras, STAT5, and phosphatidylinositol 3-kinase is required for full oncogenic activities of BCR/ABL in K562 cells. J. Biol. Chem. 2002;277:8076–8082. doi: 10.1074/jbc.M111501200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

