Olive Pomace Extract Contains Low Molecular Weight Peptides and Possesses ACE Inhibitory Activity
- PMID: 38612773
- PMCID: PMC11011677
- DOI: 10.3390/ijms25073962
Olive Pomace Extract Contains Low Molecular Weight Peptides and Possesses ACE Inhibitory Activity
Abstract
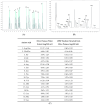
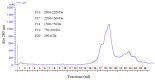
The aim of the present study was to determine the ACE inhibitory activity of aqueous extracts of olive pomace and to understand whether they represent a good source of bioactive LMW peptides for nutritional and pharmacological applications. We produced a water extract from olive pomace (var. Picual) and obtained its low molecular weight (LMW) fraction (<3 kDa). The calculated yield of extraction was 100.2 ± 7.9 mg of LMW peptides per 100 g of olive pomace. The olive pomace LMW fraction possessed strong ACE inhibitory activity (IC50 = 3.57 ± 0.22 µg prot/mL). The LMW fraction (<3 kDa) was analysed by nanoscale liquid chromatography-Orbitrap coupled with tandem mass spectrometry and de novo sequencing. Thirty new peptides, containing between 7-17 amino acids and molecular masses ranging 778-1354 Da, were identified by the Peaks database algorithm using the available Olea europaea (cv. Farga) genome database. Ten new peptides were also identified by Peaks de novo sequencing. The protein sources of twelve peptides detected in the database by Peaks DB were identified by BLAST search. The ACE inhibitory activity of the identified peptides was predicted by BIOPEP software. We conclude that olive pomace possesses ACE inhibitory activity and contains low molecular weight peptides with (predicted) biological activity. Olive pomace may represent a good source of peptides for nutritional and pharmaceutical applications. In our study, it has been shown that olive pomace possesses ACE inhibitory activity and contains low molecular weight peptides with (predicted) biological activity. Olive pomace may represent a good source of peptides for nutritional and pharmaceutical applications. More research is needed in order to identify the in vivo effects of olive pomace bioactive peptides.
Keywords: angiotensin converting enzyme (ACE); bioactive peptides; food peptides; olive pomace; peptidomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Characterisation of Endogenous Peptides Present in Virgin Olive Oil.Int J Mol Sci. 2022 Feb 2;23(3):1712. doi: 10.3390/ijms23031712. Int J Mol Sci. 2022. PMID: 35163634 Free PMC article.
-
Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia.J Nutr Health Aging. 2015 Jan;19(1):77-86. doi: 10.1007/s12603-014-0480-x. J Nutr Health Aging. 2015. PMID: 25560820 Free PMC article. Clinical Trial.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Exploration of inhibitor effect of Gly-Pro (GP), Arg-Gly-Asp-Ser (RGDS) and Ser-Asp-Gly-Arg-Gly (SDGRG) bioactive peptides on angiotensin-converting enzyme activity purified from human serum.J Biomol Struct Dyn. 2025 Jul;43(10):4901-4909. doi: 10.1080/07391102.2024.2306195. Epub 2024 Jan 21. J Biomol Struct Dyn. 2025. PMID: 38247271
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
Cited by
-
A Functional Beverage from Coffee and Olive Pomace: Polyphenol-Flavonoid Content, Antioxidant, Antihyperglycemic Properties, and Mouse Behavior.Foods. 2025 Apr 11;14(8):1331. doi: 10.3390/foods14081331. Foods. 2025. PMID: 40282734 Free PMC article.
-
Plant-Derived as Alternatives to Animal-Derived Bioactive Peptides: A Review of the Preparation, Bioactivities, Structure-Activity Relationships, and Applications in Chronic Diseases.Nutrients. 2024 Sep 27;16(19):3277. doi: 10.3390/nu16193277. Nutrients. 2024. PMID: 39408244 Free PMC article. Review.
-
Study on Fermentation Preparation, Stability, and Angiotensin-Converting Enzyme Inhibitory Activity of Tomato Pomace Peptide.Foods. 2025 Jan 7;14(2):145. doi: 10.3390/foods14020145. Foods. 2025. PMID: 39856812 Free PMC article.
References
-
- Rivas-Garcia L., Navarro-Hortal M.D., Romero-Marquez J.M., Llopis J., Forbes-Hernández T.Y., Xiao J., Quiles J.L., Sanchez-Gonzalez C. Valorization of Olea europaea and olive oil processing by-products/wastes. Adv. Food Nutr. Res. 2023;107:193–212. - PubMed
-
- Alburquerque J.A., Gonzálvez J., García D., Cegarra J. Agrochemical characterisation of ‘‘alperujo’’, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004;91:195–200. - PubMed
-
- Clemente A., Sánchez-Vioque R., Vioque J., Bautista J., Millán F. Chemical composition of extracted dried olive pomaces containing two and three phases. Food Biotechnol. 1997;11:273–291. doi: 10.1080/08905439709549936. - DOI
-
- Karpouzas D.G., Ntougias S., Iskidou E., Rousidou C., Papadopoulou K.K., Zervakis G., Ehaliotis C. Olive mill wastewater affects the structure of soil bacterial communities. Appl. Soil Ecol. 2010;45:101–111. doi: 10.1016/j.apsoil.2010.03.002. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

