Thymic Stromal Lymphopoietin (TSLP) Is Cleaved by Human Mast Cell Tryptase and Chymase
- PMID: 38612858
- PMCID: PMC11012384
- DOI: 10.3390/ijms25074049
Thymic Stromal Lymphopoietin (TSLP) Is Cleaved by Human Mast Cell Tryptase and Chymase
Abstract
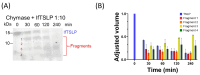
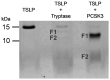
Thymic stromal lymphopoietin (TSLP), mainly expressed by epithelial cells, plays a central role in asthma. In humans, TSLP exists in two variants: the long form TSLP (lfTSLP) and a shorter TSLP isoform (sfTSLP). Macrophages (HLMs) and mast cells (HLMCs) are in close proximity in the human lung and play key roles in asthma. We evaluated the early proteolytic effects of tryptase and chymase released by HLMCs on TSLP by mass spectrometry. We also investigated whether TSLP and its fragments generated by these enzymes induce angiogenic factor release from HLMs. Mass spectrometry (MS) allowed the identification of TSLP cleavage sites caused by tryptase and chymase. Recombinant human TSLP treated with recombinant tryptase showed the production of 1-97 and 98-132 fragments. Recombinant chymase treatment of TSLP generated two peptides, 1-36 and 37-132. lfTSLP induced the release of VEGF-A, the most potent angiogenic factor, from HLMs. By contrast, the four TSLP fragments generated by tryptase and chymase failed to activate HLMs. Long-term TSLP incubation with furin generated two peptides devoid of activating property on HLMs. These results unveil an intricate interplay between mast cell-derived proteases and TSLP. These findings have potential relevance in understanding novel aspects of asthma pathobiology.
Keywords: TSLP; VEGF-A; airway remodeling; asthma; chymase; epithelial cells; macrophage; mast cell; tryptase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
TSLP is localized in and released from human lung macrophages activated by T2-high and T2-low stimuli: relevance in asthma and COPD.Eur J Intern Med. 2024 Jun;124:89-98. doi: 10.1016/j.ejim.2024.02.020. Epub 2024 Feb 24. Eur J Intern Med. 2024. PMID: 38402021
-
Human Lung-Resident Macrophages Express and Are Targets of Thymic Stromal Lymphopoietin in the Tumor Microenvironment.Cells. 2021 Aug 6;10(8):2012. doi: 10.3390/cells10082012. Cells. 2021. PMID: 34440780 Free PMC article.
-
Thymic stromal lymphopoietin (TSLP) is a substrate for tryptase in patients with mastocytosis.Eur J Intern Med. 2023 Nov;117:111-118. doi: 10.1016/j.ejim.2023.07.026. Epub 2023 Jul 26. Eur J Intern Med. 2023. PMID: 37500310
-
Thymic Stromal Lymphopoietin (TSLP), Its Isoforms and the Interplay with the Epithelium in Allergy and Asthma.Int J Mol Sci. 2023 Aug 12;24(16):12725. doi: 10.3390/ijms241612725. Int J Mol Sci. 2023. PMID: 37628907 Free PMC article. Review.
-
Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma.Int J Mol Sci. 2018 Apr 18;19(4):1231. doi: 10.3390/ijms19041231. Int J Mol Sci. 2018. PMID: 29670037 Free PMC article. Review.
Cited by
-
TSLP and TSLPR Expression Levels in Peripheral Blood as Potential Biomarkers in Patients with Chronic Rhinosinusitis with Nasal Polyps.Int J Mol Sci. 2025 Jan 30;26(3):1227. doi: 10.3390/ijms26031227. Int J Mol Sci. 2025. PMID: 39940994 Free PMC article.
-
The immunology of asthma.Nat Immunol. 2025 Aug;26(8):1233-1245. doi: 10.1038/s41590-025-02212-9. Epub 2025 Jul 29. Nat Immunol. 2025. PMID: 40730897 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

