A Novel Fibrin Matrix Derived from Platelet-Rich Plasma: Protocol and Characterization
- PMID: 38612879
- PMCID: PMC11012499
- DOI: 10.3390/ijms25074069
A Novel Fibrin Matrix Derived from Platelet-Rich Plasma: Protocol and Characterization
Abstract
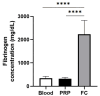
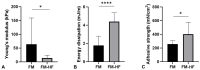
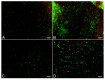
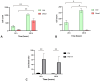
Although fibrin matrices derived from Platelet-Rich Plasma (PRP) are widely used in regenerative medicine, they have some limitations that can hinder their application. Modifying the composition of the PRP-derived fibrin matrix may improve its properties, making it suitable for certain medical uses. Three types of fibrin matrices were obtained: a PRP-derived fibrin matrix (FM), a PRP-derived fibrin matrix with a high fibrinogen content and platelets (FM-HFP) and a PRP-derived fibrin matrix with a high fibrinogen content (FM-HF). The fibrinogen levels, biomechanical properties and cell behavior were analyzed. The presence of platelets in the FM-HFP generated an inconsistent fibrin matrix that was discarded for the rest of the analysis. The fibrinogen levels in the FM-FH were higher than those in the FM (p < 0.0001), with a concentration factor of 6.86 ± 1.81. The values of clotting and swelling achieved using the FM-HF were higher (p < 0.0001), with less clot shrinkage (p < 0.0001). The FM had a significantly higher stiffness and turned out to be the most adherent composition (p = 0.027). In terms of cell viability, the FM-HF showed less cell proliferation but higher live/dead ratio values (p < 0.01). The increased fibrinogen and platelet removal in the FM-HF improved its adhesion and other biomechanical properties without affecting cell viability.
Keywords: fibrin; fibrinogen; growth factors; matrix; platelet-rich plasma; platelets; scaffold.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
Figures


References
-
- Coulange Zavarro A., De Girolamo L., Laver L., Sánchez M., Tischer T., Filardo G., Sabatier F., Magalon J. The Top 100 Most Cited Articles on Platelet-Rich Plasma Use in Regenerative Medicine-A Bibliometric Analysis-From the ESSKA Orthobiologic Initiative. Bioengineering. 2022;9:580. doi: 10.3390/bioengineering9100580. - DOI - PMC - PubMed
-
- Weisel J.W. Advances in Protein Chemistry. Volume 70. Elsevier; Amsterdam, The Netherlands: 2005. Fibrinogen and Fibrin; pp. 247–299. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

