Calcium and Neural Stem Cell Proliferation
- PMID: 38612887
- PMCID: PMC11012558
- DOI: 10.3390/ijms25074073
Calcium and Neural Stem Cell Proliferation
Abstract
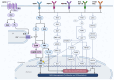
Intracellular calcium plays a pivotal role in central nervous system (CNS) development by regulating various processes such as cell proliferation, migration, differentiation, and maturation. However, understanding the involvement of calcium (Ca2+) in these processes during CNS development is challenging due to the dynamic nature of this cation and the evolving cell populations during development. While Ca2+ transient patterns have been observed in specific cell processes and molecules responsible for Ca2+ homeostasis have been identified in excitable and non-excitable cells, further research into Ca2+ dynamics and the underlying mechanisms in neural stem cells (NSCs) is required. This review focuses on molecules involved in Ca2+ entrance expressed in NSCs in vivo and in vitro, which are crucial for Ca2+ dynamics and signaling. It also discusses how these molecules might play a key role in balancing cell proliferation for self-renewal or promoting differentiation. These processes are finely regulated in a time-dependent manner throughout brain development, influenced by extrinsic and intrinsic factors that directly or indirectly modulate Ca2+ dynamics. Furthermore, this review addresses the potential implications of understanding Ca2+ dynamics in NSCs for treating neurological disorders. Despite significant progress in this field, unraveling the elements contributing to Ca2+ intracellular dynamics in cell proliferation remains a challenging puzzle that requires further investigation.
Keywords: calcium signaling; differentiation; neural stem/progenitor cells; proliferation; radial glial cells.
Conflict of interest statement
The authors declare no conflicts of interest in the writing of the manuscript.
Figures
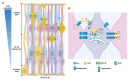
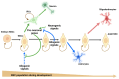
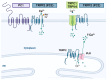
 ) to increase their pool or asymmetrically (black) to give rise, directly or indirectly, in a time-dependent manner, to neurons (green arrows), astrocytes (blue arrows), and oligodendrocytes (red arrow). NSCs = neural stem cells; IPCs = intermediate progenitor cells. Blue doted arrow = repressed gliogenic signals; Blunt green arrow = pro-neuronal genes repressing gliogenic signals; Blunt blue arrow = gliogenic signals repressing neuronal genes. Created using
) to increase their pool or asymmetrically (black) to give rise, directly or indirectly, in a time-dependent manner, to neurons (green arrows), astrocytes (blue arrows), and oligodendrocytes (red arrow). NSCs = neural stem cells; IPCs = intermediate progenitor cells. Blue doted arrow = repressed gliogenic signals; Blunt green arrow = pro-neuronal genes repressing gliogenic signals; Blunt blue arrow = gliogenic signals repressing neuronal genes. Created using 
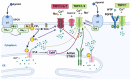
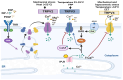
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

