The Intricate Balance between Life and Death: ROS, Cathepsins, and Their Interplay in Cell Death and Autophagy
- PMID: 38612897
- PMCID: PMC11012956
- DOI: 10.3390/ijms25074087
The Intricate Balance between Life and Death: ROS, Cathepsins, and Their Interplay in Cell Death and Autophagy
Abstract
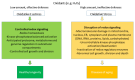
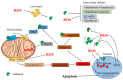
Cellular survival hinges on a delicate balance between accumulating damages and repair mechanisms. In this intricate equilibrium, oxidants, currently considered physiological molecules, can compromise vital cellular components, ultimately triggering cell death. On the other hand, cells possess countermeasures, such as autophagy, which degrades and recycles damaged molecules and organelles, restoring homeostasis. Lysosomes and their enzymatic arsenal, including cathepsins, play critical roles in this balance, influencing the cell's fate toward either apoptosis and other mechanisms of regulated cell death or autophagy. However, the interplay between reactive oxygen species (ROS) and cathepsins in these life-or-death pathways transcends a simple cause-and-effect relationship. These elements directly and indirectly influence each other's activities, creating a complex web of interactions. This review delves into the inner workings of regulated cell death and autophagy, highlighting the pivotal role of ROS and cathepsins in these pathways and their intricate interplay.
Keywords: apoptosis; autophagy; cathepsins; cell death; oxidative stress; reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Lysosomes and lysosomal cathepsins in cell death.Biochim Biophys Acta. 2012 Jan;1824(1):22-33. doi: 10.1016/j.bbapap.2011.08.016. Epub 2011 Sep 3. Biochim Biophys Acta. 2012. PMID: 21914490 Review.
-
Autophagy is activated and involved in cell death with participation of cathepsins during stress-induced microspore embryogenesis in barley.J Exp Bot. 2018 Mar 14;69(6):1387-1402. doi: 10.1093/jxb/erx455. J Exp Bot. 2018. PMID: 29309624 Free PMC article.
-
Autophagy induced by cathepsin S inhibition induces early ROS production, oxidative DNA damage, and cell death via xanthine oxidase.Free Radic Biol Med. 2013 Dec;65:1473-1486. doi: 10.1016/j.freeradbiomed.2013.07.020. Epub 2013 Jul 25. Free Radic Biol Med. 2013. PMID: 23892358
-
Pro-Death or Pro-Survival: Contrasting Paradigms on Nanomaterial-Induced Autophagy and Exploitations for Cancer Therapy.Acc Chem Res. 2019 Nov 19;52(11):3164-3176. doi: 10.1021/acs.accounts.9b00397. Epub 2019 Oct 17. Acc Chem Res. 2019. PMID: 31621285 Review.
-
Autophagy-Regulated ROS from Xanthine Oxidase Acts as an Early Effector for Triggering Late Mitochondria-Dependent Apoptosis in Cathepsin S-Targeted Tumor Cells.PLoS One. 2015 Jun 1;10(6):e0128045. doi: 10.1371/journal.pone.0128045. eCollection 2015. PLoS One. 2015. PMID: 26029922 Free PMC article.
Cited by
-
Autophagy and Female Fertility: Mechanisms, Clinical Implications, and Emerging Therapies.Cells. 2024 Aug 14;13(16):1354. doi: 10.3390/cells13161354. Cells. 2024. PMID: 39195244 Free PMC article. Review.
-
Review of Cathepsin K Inhibitor Development and the Potential Role of Phytochemicals.Molecules. 2024 Dec 29;30(1):91. doi: 10.3390/molecules30010091. Molecules. 2024. PMID: 39795149 Free PMC article. Review.
-
Gold Nanoparticles as a Platform for Delivery of Immunogenic Peptides to THP-1 Derived Macrophages: Insights into Nanotoxicity.Vaccines (Basel). 2025 Jan 24;13(2):119. doi: 10.3390/vaccines13020119. Vaccines (Basel). 2025. PMID: 40006666 Free PMC article.
-
Cathepsin B induces kidney diseases through different types of programmed cell death.Front Immunol. 2025 Mar 10;16:1535313. doi: 10.3389/fimmu.2025.1535313. eCollection 2025. Front Immunol. 2025. PMID: 40129990 Free PMC article. Review.
-
From Nutrient to Nanocarrier: The Multifaceted Role of Vitamin B12 in Drug Delivery.Int J Mol Sci. 2025 May 26;26(11):5119. doi: 10.3390/ijms26115119. Int J Mol Sci. 2025. PMID: 40507930 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

