Agnostic Administration of Targeted Anticancer Drugs: Looking for a Balance between Hype and Caution
- PMID: 38612902
- PMCID: PMC11012409
- DOI: 10.3390/ijms25074094
Agnostic Administration of Targeted Anticancer Drugs: Looking for a Balance between Hype and Caution
Abstract
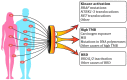
Many tumors have well-defined vulnerabilities, thus potentially allowing highly specific and effective treatment. There is a spectrum of actionable genetic alterations which are shared across various tumor types and, therefore, can be targeted by a given drug irrespective of tumor histology. Several agnostic drug-target matches have already been approved for clinical use, e.g., immune therapy for tumors with microsatellite instability (MSI) and/or high tumor mutation burden (TMB), NTRK1-3 and RET inhibitors for cancers carrying rearrangements in these kinases, and dabrafenib plus trametinib for BRAF V600E mutated malignancies. Multiple lines of evidence suggest that this histology-independent approach is also reasonable for tumors carrying ALK and ROS1 translocations, biallelic BRCA1/2 inactivation and/or homologous recombination deficiency (HRD), strong HER2 amplification/overexpression coupled with the absence of other MAPK pathway-activating mutations, etc. On the other hand, some well-known targets are not agnostic: for example, PD-L1 expression is predictive for the efficacy of PD-L1/PD1 inhibitors only in some but not all cancer types. Unfortunately, the individual probability of finding a druggable target in a given tumor is relatively low, even with the use of comprehensive next-generation sequencing (NGS) assays. Nevertheless, the rapidly growing utilization of NGS will significantly increase the number of patients with highly unusual or exceptionally rare tumor-target combinations. Clinical trials may provide only a framework for treatment attitudes, while the decisions for individual patients usually require case-by-case consideration of the probability of deriving benefit from agnostic versus standard therapy, drug availability, associated costs, and other circumstances. The existing format of data dissemination may not be optimal for agnostic cancer medicine, as conventional scientific journals are understandably biased towards the publication of positive findings and usually discourage the submission of case reports. Despite all the limitations and concerns, histology-independent drug-target matching is certainly feasible and, therefore, will be increasingly utilized in the future.
Keywords: HRD; TMB; agnostic markers; kinase inhibitors; microsatellite instability; targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Target-Driven Tissue-Agnostic Drug Approvals-A New Path of Drug Development.Cancers (Basel). 2024 Jul 13;16(14):2529. doi: 10.3390/cancers16142529. Cancers (Basel). 2024. PMID: 39061168 Free PMC article. Review.
-
Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer.Cancer Med. 2021 Apr;10(7):2216-2231. doi: 10.1002/cam4.3649. Epub 2021 Mar 2. Cancer Med. 2021. PMID: 33655698 Free PMC article.
-
ALK, ROS1, RET and NTRK1-3 Gene Fusions in Colorectal and Non-Colorectal Microsatellite-Unstable Cancers.Int J Mol Sci. 2023 Sep 2;24(17):13610. doi: 10.3390/ijms241713610. Int J Mol Sci. 2023. PMID: 37686416 Free PMC article.
-
Cancer Therapy Guided by Mutation Tests: Current Status and Perspectives.Int J Mol Sci. 2021 Oct 10;22(20):10931. doi: 10.3390/ijms222010931. Int J Mol Sci. 2021. PMID: 34681592 Free PMC article. Review.
-
Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer.J Immunother Cancer. 2021 Aug;9(8):e002891. doi: 10.1136/jitc-2021-002891. J Immunother Cancer. 2021. PMID: 34376553 Free PMC article.
Cited by
-
The potential of ME1 in guiding immunotherapeutic strategies for ovarian cancer: insights from pan-cancer research.Front Immunol. 2025 May 29;16:1571842. doi: 10.3389/fimmu.2025.1571842. eCollection 2025. Front Immunol. 2025. PMID: 40510340 Free PMC article.
-
Trastuzumab-Deruxtecan: Redefining HER2 as a Tumor Agnostic Biomarker.Target Oncol. 2024 Sep;19(5):705-710. doi: 10.1007/s11523-024-01079-4. Epub 2024 Jul 4. Target Oncol. 2024. PMID: 38963654 Review.
-
Novel kinase-activating genetic events in non-small cell lung carcinomas.Explor Target Antitumor Ther. 2025 Jul 9;6:1002330. doi: 10.37349/etat.2025.1002330. eCollection 2025. Explor Target Antitumor Ther. 2025. PMID: 40661875 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

