Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications
- PMID: 38612904
- PMCID: PMC11012347
- DOI: 10.3390/ijms25074096
Roles of Integrin in Cardiovascular Diseases: From Basic Research to Clinical Implications
Abstract
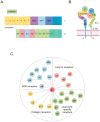
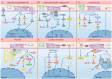
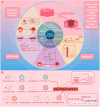
Cardiovascular diseases (CVDs) pose a significant global health threat due to their complex pathogenesis and high incidence, imposing a substantial burden on global healthcare systems. Integrins, a group of heterodimers consisting of α and β subunits that are located on the cell membrane, have emerged as key players in mediating the occurrence and progression of CVDs by regulating the physiological activities of endothelial cells, vascular smooth muscle cells, platelets, fibroblasts, cardiomyocytes, and various immune cells. The crucial role of integrins in the progression of CVDs has valuable implications for targeted therapies. In this context, the development and application of various integrin antibodies and antagonists have been explored for antiplatelet therapy and anti-inflammatory-mediated tissue damage. Additionally, the rise of nanomedicine has enhanced the specificity and bioavailability of precision therapy targeting integrins. Nevertheless, the complexity of the pathogenesis of CVDs presents tremendous challenges for monoclonal targeted treatment. This paper reviews the mechanisms of integrins in the development of atherosclerosis, cardiac fibrosis, hypertension, and arrhythmias, which may pave the way for future innovations in the diagnosis and treatment of CVDs.
Keywords: cardiac fibroblasts; cardiomyocytes; cardiovascular diseases; integrin antagonists and antibodies; integrins; nanotherapy; platelets; vascular endothelial cells; vascular smooth muscle cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Toll-Like Receptor 3 in Cardiovascular Diseases.Heart Lung Circ. 2022 Jul;31(7):e93-e109. doi: 10.1016/j.hlc.2022.02.012. Epub 2022 Mar 30. Heart Lung Circ. 2022. PMID: 35367134 Review.
-
A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes.Curr Pharm Des. 2019;25(38):4063-4084. doi: 10.2174/1381612825666190925163827. Curr Pharm Des. 2019. PMID: 31553287 Review.
-
Roles and potential clinical implications of tissue transglutaminase in cardiovascular diseases.Pharmacol Res. 2022 Mar;177:106085. doi: 10.1016/j.phrs.2022.106085. Epub 2022 Jan 13. Pharmacol Res. 2022. PMID: 35033646 Review.
-
Adhesion of activated T lymphocytes to vascular smooth muscle cells and dermal fibroblasts is mediated by beta 1- and beta 2-integrins.Scand J Immunol. 1992 Aug;36(2):233-42. doi: 10.1111/j.1365-3083.1992.tb03095.x. Scand J Immunol. 1992. PMID: 1380179
-
Recent Advances in Cell-Based Nanotherapy for Cardiovascular Diseases.Macromol Biosci. 2023 Jul;23(7):e2200537. doi: 10.1002/mabi.202200537. Epub 2023 Mar 12. Macromol Biosci. 2023. PMID: 36872633 Review.
Cited by
-
Evaluating sex-specific responses to western diet across the lifespan: impact on cardiac function and transcriptomic signatures in C57BL/6J mice at 530 and 640/750 days of age.Cardiovasc Diabetol. 2024 Dec 28;23(1):454. doi: 10.1186/s12933-024-02565-9. Cardiovasc Diabetol. 2024. PMID: 39732652 Free PMC article.
-
Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases.Biosensors (Basel). 2025 Apr 5;15(4):232. doi: 10.3390/bios15040232. Biosensors (Basel). 2025. PMID: 40277546 Free PMC article. Review.
-
RGD peptide-functionalized micelles loaded with crocetin ameliorate doxorubicin-induced cardiotoxicity.Int J Pharm X. 2025 Feb 26;9:100326. doi: 10.1016/j.ijpx.2025.100326. eCollection 2025 Jun. Int J Pharm X. 2025. PMID: 40103672 Free PMC article.
-
Environmental Toxicants and Their Disruption of Integrin Signaling in Lipid Rafts.Bioessays. 2025 May;47(5):e202400276. doi: 10.1002/bies.202400276. Epub 2025 Feb 26. Bioessays. 2025. PMID: 40012268 Free PMC article. Review.
References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Alonso A., Beaton A.Z., Bittencourt M.S., Boehme A.K., Buxton A.E., Carson A.P., Commodore-Mensah Y., et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/cir.0000000000001052. - DOI - PubMed
-
- Wulf-Johansson H., Lock Johansson S., Schlosser A., Trommelholt Holm A., Rasmussen L.M., Mickley H., Diederichsen A.C., Munkholm H., Poulsen T.S., Tornøe I., et al. Localization of microfibrillar-associated protein 4 (MFAP4) in human tissues: Clinical evaluation of serum MFAP4 and its association with various cardiovascular conditions. PLoS ONE. 2013;8:e82243. doi: 10.1371/journal.pone.0082243. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

