Informed by Cancer Stem Cells of Solid Tumors: Advances in Treatments Targeting Tumor-Promoting Factors and Pathways
- PMID: 38612911
- PMCID: PMC11012648
- DOI: 10.3390/ijms25074102
Informed by Cancer Stem Cells of Solid Tumors: Advances in Treatments Targeting Tumor-Promoting Factors and Pathways
Abstract
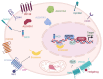
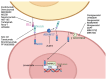
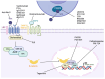
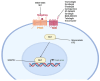
Cancer stem cells (CSCs) represent a subpopulation within tumors that promote cancer progression, metastasis, and recurrence due to their self-renewal capacity and resistance to conventional therapies. CSC-specific markers and signaling pathways highly active in CSCs have emerged as a promising strategy for improving patient outcomes. This review provides a comprehensive overview of the therapeutic targets associated with CSCs of solid tumors across various cancer types, including key molecular markers aldehyde dehydrogenases, CD44, epithelial cellular adhesion molecule, and CD133 and signaling pathways such as Wnt/β-catenin, Notch, and Sonic Hedgehog. We discuss a wide array of therapeutic modalities ranging from targeted antibodies, small molecule inhibitors, and near-infrared photoimmunotherapy to advanced genetic approaches like RNA interference, CRISPR/Cas9 technology, aptamers, antisense oligonucleotides, chimeric antigen receptor (CAR) T cells, CAR natural killer cells, bispecific T cell engagers, immunotoxins, drug-antibody conjugates, therapeutic peptides, and dendritic cell vaccines. This review spans developments from preclinical investigations to ongoing clinical trials, highlighting the innovative targeting strategies that have been informed by CSC-associated pathways and molecules to overcome therapeutic resistance. We aim to provide insights into the potential of these therapies to revolutionize cancer treatment, underscoring the critical need for a multi-faceted approach in the battle against cancer. This comprehensive analysis demonstrates how advances made in the CSC field have informed significant developments in novel targeted therapeutic approaches, with the ultimate goal of achieving more effective and durable responses in cancer patients.
Keywords: cancer stem cells; clinical trial; marker; target; therapeutic.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection; analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish.
Figures
Similar articles
-
Update on immune-based therapy strategies targeting cancer stem cells.Cancer Med. 2023 Sep;12(18):18960-18980. doi: 10.1002/cam4.6520. Epub 2023 Sep 12. Cancer Med. 2023. PMID: 37698048 Free PMC article. Review.
-
Cancer Stem Cell Regulation as a Target of Therapeutic Intervention: Insights into Breast, Cervical and Lung Cancer.Cell Biochem Biophys. 2025 Jun;83(2):1521-1535. doi: 10.1007/s12013-025-01666-w. Epub 2025 Jan 23. Cell Biochem Biophys. 2025. PMID: 39843681 Review.
-
Breaking Immunosuppression to Enhance Cancer Stem Cell-Targeted Immunotherapy.Int J Biol Sci. 2025 Feb 10;21(4):1819-1836. doi: 10.7150/ijbs.101025. eCollection 2025. Int J Biol Sci. 2025. PMID: 39990669 Free PMC article. Review.
-
Targeting cancer stem cell-specific markers and/or associated signaling pathways for overcoming cancer drug resistance.Tumour Biol. 2016 Oct;37(10):13059-13075. doi: 10.1007/s13277-016-5294-5. Epub 2016 Aug 26. Tumour Biol. 2016. PMID: 27561758 Review.
-
Cancer stem cells (CSCs) in cancer progression and therapy.J Cell Physiol. 2019 Jun;234(6):8381-8395. doi: 10.1002/jcp.27740. Epub 2018 Nov 11. J Cell Physiol. 2019. PMID: 30417375 Review.
Cited by
-
Impact of G1 phase kinetics on the acquisition of stemness in cancer cells: the critical role of cyclin D.Mol Biol Rep. 2025 Feb 14;52(1):230. doi: 10.1007/s11033-025-10351-3. Mol Biol Rep. 2025. PMID: 39951181 Review.
-
Targeting cancer stem cells with CAR-based immunotherapy: biology, evidence, and future directions.Cancer Cell Int. 2025 Jul 28;25(1):289. doi: 10.1186/s12935-025-03846-3. Cancer Cell Int. 2025. PMID: 40722155 Free PMC article. Review.
-
Personalized Treatment Strategies via Integration of Gene Expression Biomarkers in Molecular Profiling of Laryngeal Cancer.J Pers Med. 2024 Oct 10;14(10):1048. doi: 10.3390/jpm14101048. J Pers Med. 2024. PMID: 39452555 Free PMC article. Review.
-
Calpain 2 promotes Lenvatinib resistance and cancer stem cell traits via both proteolysis-dependent and independent approach in hepatocellular carcinoma.Mol Biomed. 2024 Dec 31;5(1):74. doi: 10.1186/s43556-024-00242-7. Mol Biomed. 2024. PMID: 39739077 Free PMC article.
-
Wnt signaling aberrant activation drives ameloblastoma invasion and recurrence: bioinformatics and in vitro insights.BMC Oral Health. 2024 Nov 21;24(1):1421. doi: 10.1186/s12903-024-05003-0. BMC Oral Health. 2024. PMID: 39574093 Free PMC article.
References
-
- Ginestier C., Hur M.H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., Jacquemier J., Viens P., Kleer C.G., Liu S., et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

