Tumor and Peritoneum-Associated Macrophage Gene Signature as a Novel Molecular Biomarker in Gastric Cancer
- PMID: 38612926
- PMCID: PMC11012629
- DOI: 10.3390/ijms25074117
Tumor and Peritoneum-Associated Macrophage Gene Signature as a Novel Molecular Biomarker in Gastric Cancer
Abstract
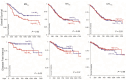
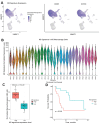
A spectrum of immune states resulting from tumor resident macrophages and T-lymphocytes in the solid tumor microenvironment correlates with patient outcomes. We hypothesized that in gastric cancer (GC), macrophages in a polarized immunosuppressive transcriptional state would be prognostic of poor survival. We derived transcriptomic signatures for M2 (M2TS, MRC1; MS4A4A; CD36; CCL13; CCL18; CCL23; SLC38A6; FGL2; FN1; MAF) and M1 (M1TS, CCR7; IL2RA; CXCL11; CCL19; CXCL10; PLA1A; PTX3) macrophages, and cytolytic T-lymphocytes (CTLTS, GZMA; GZMB; GZMH; GZMM; PRF1). Primary GC in a TCGA stomach cancer dataset was evaluated for signature expressions, and a log-rank test determined overall survival (OS) and the disease-free interval (DFI). In 341 TCGA GC entries, high M2TS expression was associated with histological types and later stages. Low M2TS expression was associated with significantly better 5-year OS and DFI. We validated M2TS in prospectively collected peritoneal fluid of a GC patient cohort (n = 28). Single-cell RNA sequencing was used for signature expression in CD68+CD163+ cells and the log-rank test compared OS. GC patients with high M2TS in CD68+CD163+ cells in their peritoneal fluid had significantly worse OS than those with low expression. Multivariate analyses confirmed M2TS was significantly and independently associated with survival. As an independent predictor of poor survival, M2TS may be prognostic in primary tumors and peritoneal fluid of GC patients.
Keywords: biomarker; gastric cancer; liquid biopsy; macrophage; peritoneal metastases.
Conflict of interest statement
Von Hoff—Employment at McKesson; Stock and other ownership interests in Medtronic, CerRx, SynRevRx, United Healthcare, Anthem Inc., Stromatis Pharma, Systems Oncology, Stingray Therapeutics, FORMA Therapeutics, Orpheus Bioscience, AADi, and Origin Commercial Advisors; Consulting or advisory role at DNAtrix, Imaging Endpoints, Immodulon Therapeutics, Senhwa Bioscience, Tolero Pharmaceuticals, Alpha Cancer Technologies, CanBas, Lixte Biotechnology, Oncolyze, RenovoRx, TD2, Aptose Bioscience, CV6 Therapeutics, EMD Serono, Fujifilm, Phosplatin Therapeutics, SOTIO, Synergene, Geistlich Pharma, HUYA Bioscience International, Immunophotonics, Genzada Pharmaceuticals, L.E.A.F. Pharmaceuticals, Oncology Venture, Verily, Athenex, Novita Pharmaceuticals, Victus Therapeutics, Codiak Bioscience, Agenus, RadImmune, Samumed, BioXCel Therapeutics, Bryologyx, Sirnaomics, AiMed, Corcept Therapeutics, Erimos Pharmaceuticals, Gimbal, Pfizer, GiraFPharma, Axis Therapeutics, ImmuneOncia, Orphagen Pharmaceuticals, Viracta Pharmaceuticals, AlaMab Therapeutics, Avesta76 Therapeutics, NeoTx, Xerient, Decoy Biosystems, Noxxon Pharma, Reflexion Medical, Reglagene, Lycia Therapeutics, NGM Biopharmaceuticals, Coordination pharmaceuticals, EXACT Therapeutics, Nirogy Therapeutics, Seattle Genetics, Agastiya Biotech, Amunix, Atalion Therapeutics, Cytocom, GlaxoSmithKline, ImaginAb, Signablok, SonaCare Medical, Caribou Bioscience, Xenter, Compass Therapeutics, Vivacitas Oncology, Sumitomo Dainippon Pharma Oncology, OnQuality Pharmaceuticals, Sellas Life Sciences, Catamaran Bio, and Thirona Bioscience; Research funding from Lilly, Genentech, Celgene, Incyte, Merrimack, Plexxikon, Minneamrita Therapeutics, Abbvie, Aduro Biotech, Cleave Bioscience, CytRx Corporation, Daiichi Sankyo, Deciphera, Endocyte, Exelixis, Five Prime Therapeutics, Gilead Sciences, Merck, Pfizer, Pharmacyclics, Phoenix Biotech, Samumed, Strategia, and Halozyme. Fong—Paid scientific consultant for Medtronics, Johnson & Johnson, and Imugene; Receives royalties for inventions from Merck and Imugene. Woo—Scientific advisor for Imugene and J&J Ethicon. All other authors have no competing interests.
Figures

References
-
- Ferlay J., Ervik M., Lam F., Laversanne M., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today (Version 1.1) International Agency for Research on Cancer; Lyon, France: 2024. [(accessed on 14 March 2024)]. Available online: https://gco.iarc.who.int/today.
-
- Sato Y., Wada I., Odaira K., Hosoi A., Kobayashi Y., Nagaoka K., Karasaki T., Matsushita H., Yagi K., Yamashita H., et al. Integrative immunogenomic analysis of gastric cancer dictates novel immunological classification and the functional status of tumor-infiltrating cells. Clin. Transl. Immunol. 2020;9:e1194. doi: 10.1002/cti2.1194. - DOI - PMC - PubMed
-
- Shitara K., Van Cutsem E., Bang Y.J., Fuchs C., Wyrwicz L., Lee K.W., Kudaba I., Garrido M., Chung H.C., Lee J., et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

