Brain-Derived Neurotrophic Factor as a Potential Mediator of the Beneficial Effects of Myo-Inositol Supplementation during Suckling in the Offspring of Gestational-Calorie-Restricted Rats
- PMID: 38613013
- PMCID: PMC11013066
- DOI: 10.3390/nu16070980
Brain-Derived Neurotrophic Factor as a Potential Mediator of the Beneficial Effects of Myo-Inositol Supplementation during Suckling in the Offspring of Gestational-Calorie-Restricted Rats
Abstract
This study aims to investigate the potential mechanisms underlying the protective effects of myo-inositol (MI) supplementation during suckling against the detrimental effects of fetal energy restriction described in animal studies, particularly focusing on the potential connections with BDNF signaling. Oral physiological doses of MI or the vehicle were given daily to the offspring of control (CON) and 25%-calorie-restricted (CR) pregnant rats during suckling. The animals were weaned and then fed a standard diet until 5 months of age, when the diet was switched to a Western diet until 7 months of age. At 25 days and 7 months of age, the plasma BDNF levels and mRNA expression were analyzed in the hypothalamus and three adipose tissue depots. MI supplementation, especially in the context of gestational calorie restriction, promoted BDNF secretion and signaling at a juvenile age and in adulthood, which was more evident in the male offspring of the CR dams than in females. Moreover, the CR animals supplemented with MI exhibited a stimulated anorexigenic signaling pathway in the hypothalamus, along with improved peripheral glucose management and enhanced browning capacity. These findings suggest a novel connection between MI supplementation during suckling, BDNF signaling, and metabolic programming, providing insights into the mechanisms underlying the beneficial effects of MI during lactation.
Keywords: BDNF; adipose tissue; browning; glucose control; hypothalamus; insulin sensitivity; metabolic programming; perinatal.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
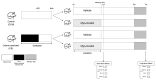

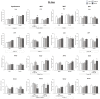
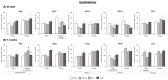

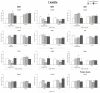

References
MeSH terms
Substances
Grants and funding
- PGC2018-097436-B-I00 and PID2022-138140NB-I00/MCIN/AEI/ 10.13039/501100011033 and ERDF A way of making Europe
- Instituto de Salud Carlos III
- Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición, CIBEROBN
- Member/European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: no. FP6-506360).
LinkOut - more resources
Full Text Sources

