Microanatomy of the human tunnel of Corti structures and cochlear partition-tonotopic variations and transcellular signaling
- PMID: 38613211
- PMCID: PMC11259753
- DOI: 10.1111/joa.14045
Microanatomy of the human tunnel of Corti structures and cochlear partition-tonotopic variations and transcellular signaling
Abstract
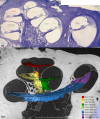
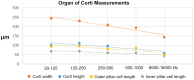
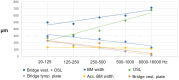
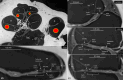
Auditory sensitivity and frequency resolution depend on the optimal transfer of sound-induced vibrations from the basilar membrane (BM) to the inner hair cells (IHCs), the principal auditory receptors. There remains a paucity of information on how this is accomplished along the frequency range in the human cochlea. Most of the current knowledge is derived either from animal experiments or human tissue processed after death, offering limited structural preservation and optical resolution. In our study, we analyzed the cytoarchitecture of the human cochlear partition at different frequency locations using high-resolution microscopy of uniquely preserved normal human tissue. The results may have clinical implications and increase our understanding of how frequency-dependent acoustic vibrations are carried to human IHCs. A 1-micron-thick plastic-embedded section (mid-modiolar) from a normal human cochlea uniquely preserved at lateral skull base surgery was analyzed using light and transmission electron microscopy (LM, TEM). Frequency locations were estimated using synchrotron radiation phase-contrast imaging (SR-PCI). Archival human tissue prepared for scanning electron microscopy (SEM) and super-resolution structured illumination microscopy (SR-SIM) were also used and compared in this study. Microscopy demonstrated great variations in the dimension and architecture of the human cochlear partition along the frequency range. Pillar cell geometry was closely regulated and depended on the reticular lamina slope and tympanic lip angle. A type II collagen-expressing lamina extended medially from the tympanic lip under the inner sulcus, here named "accessory basilar membrane." It was linked to the tympanic lip and inner pillar foot, and it may contribute to the overall compliance of the cochlear partition. Based on the findings, we speculate on the remarkable microanatomic inflections and geometric relationships which relay different sound-induced vibrations to the IHCs, including their relevance for the evolution of human speech reception and electric stimulation with auditory implants. The inner pillar transcellular microtubule/actin system's role of directly converting vibration energy to the IHC cuticular plate and ciliary bundle is highlighted.
Keywords: cochlea; human; microanatomy; microtubules; synchrotron radiation phase‐contrast imaging.
© 2024 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Disclosures: The Western University Foundation has received a donation from Med‐El GmbH. However, this work was carried out independent of industry involvement or oversight.
Figures












Similar articles
-
An outer hair cell-powered global hydromechanical mechanism for cochlear amplification.Hear Res. 2022 Sep 15;423:108407. doi: 10.1016/j.heares.2021.108407. Epub 2021 Dec 1. Hear Res. 2022. PMID: 34922772 Free PMC article.
-
Two-compartment passive frequency domain cochlea model allowing independent fluid coupling to the tectorial and basilar membranes.J Acoust Soc Am. 2015 Mar;137(3):1117-25. doi: 10.1121/1.4908214. J Acoust Soc Am. 2015. PMID: 25786927 Free PMC article.
-
Direct visualization of organ of corti kinematics in a hemicochlea.J Neurophysiol. 1999 Nov;82(5):2798-807. doi: 10.1152/jn.1999.82.5.2798. J Neurophysiol. 1999. PMID: 10561446
-
Anatomical differences in the peripheral auditory system of mammals and man. A mini review.Adv Otorhinolaryngol. 2002;59:1-10. doi: 10.1159/000059235. Adv Otorhinolaryngol. 2002. PMID: 11885648 Review.
-
Frequency tuning of mechanical responses in the mammalian cochlea.Biol Res. 1996;29(3):325-31. Biol Res. 1996. PMID: 9278704 Review.
Cited by
-
Morphology of the human inner ear and vestibulocochlear nerve assessed using 7 T MRI.MAGMA. 2025 Feb;38(1):121-130. doi: 10.1007/s10334-024-01213-3. Epub 2024 Nov 13. MAGMA. 2025. PMID: 39535680 Free PMC article.
-
Immuno-surveillance and protection of the human cochlea.Front Neurol. 2024 May 16;15:1355785. doi: 10.3389/fneur.2024.1355785. eCollection 2024. Front Neurol. 2024. PMID: 38817543 Free PMC article.
References
-
- Angelborg, C. & Engström, H. (1972) Supporting elements in the organ of Corti I. Fibrillar structures in the supporting cells of the organ of Corti of mammals. Acta Oto‐Laryngologica, 73, 49–60. - PubMed
-
- Arnold, W. (1987) Myelination of the human spiral ganglion. Acta Oto‐Laryngologica, 104, 76–84. - PubMed
-
- Békésy, G. (1960) Experiments in hearing. New York, NY: McGraw‐Hill.
-
- Bieniussa, L. , Jain, I. , Bosch Grau, M. , Juergens, L. , Hagen, R. , Janke, C. et al. (2023) Microtubule and auditory function – an underestimated connection. Seminars in Cell & Developmental Biology, 137, 74–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

