Liposomal Phenylephrine Nanoparticles Enhance the Antitumor Activity of Intratumoral Chemotherapy in a Preclinical Model of Melanoma
- PMID: 38613483
- PMCID: PMC11301277
- DOI: 10.1021/acsbiomaterials.4c00078
Liposomal Phenylephrine Nanoparticles Enhance the Antitumor Activity of Intratumoral Chemotherapy in a Preclinical Model of Melanoma
Abstract
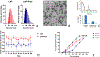
Intratumoral injection of anticancer agents has limited efficacy and is not routinely used for most cancers. In this study, we aimed to improve the efficacy of intratumoral chemotherapy using a novel approach comprising peri-tumoral injection of sustained-release liposomal nanoparticles containing phenylephrine, which is a potent vasoconstrictor. Using a preclinical model of melanoma, we have previously shown that systemically administered (intravenous) phenylephrine could transiently shunt blood flow to the tumor at the time of drug delivery, which in turn improved antitumor responses. This approach was called dynamic control of tumor-associated vessels. Herein, we used liposomal phenylephrine nanoparticles as a "local" dynamic control strategy for the B16 melanoma. Local dynamic control was shown to increase the retention and exposure time of tumors to intratumorally injected chemotherapy (melphalan). C57BL/6 mice bearing B16 tumors were treated with intratumoral melphalan and peri-tumoral injection of sustained-release liposomal phenylephrine nanoparticles (i.e., the local dynamic control protocol). These mice had statistically significantly improved antitumor responses compared to melphalan alone (p = 0.0011), whereby 58.3% obtained long-term complete clinical response. Our novel approach of local dynamic control demonstrated significantly enhanced antitumor efficacy and is the subject of future clinical trials being designed by our group.
Keywords: cancer therapies; drug delivery; tumor vessels.
Conflict of interest statement
Figures

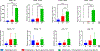

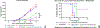
Similar articles
-
Dynamic control of tumor vasculature improves antitumor responses in a regional model of melanoma.Sci Rep. 2020 Aug 6;10(1):13245. doi: 10.1038/s41598-020-70233-5. Sci Rep. 2020. PMID: 32764623 Free PMC article.
-
The Effect of Phase Transition Temperature on Therapeutic Efficacy of Liposomal Bortezomib.Anticancer Agents Med Chem. 2020;20(6):700-708. doi: 10.2174/1871520620666200101150640. Anticancer Agents Med Chem. 2020. PMID: 31893998
-
Anti-tumor activity of liposomal glucocorticoids: The relevance of liposome-mediated drug delivery, intratumoral localization and systemic activity.J Control Release. 2011 Apr 10;151(1):10-7. doi: 10.1016/j.jconrel.2010.11.031. Epub 2010 Dec 3. J Control Release. 2011. PMID: 21130819
-
Hyperthermia potentiates antitumor effect of thermosensitive-liposome-encapsulated melphalan and radiation in murine melanoma.Tumour Biol. 1997;18(4):250-60. doi: 10.1159/000218038. Tumour Biol. 1997. PMID: 9218010
-
Therapeutic Potential of Silver Nitroprusside Nanoparticles for Melanoma.ACS Appl Bio Mater. 2024 Aug 19;7(8):5057-5075. doi: 10.1021/acsabm.4c00597. Epub 2024 Aug 8. ACS Appl Bio Mater. 2024. PMID: 39115261 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
