Liposomes to Cubosomes: The Evolution of Lipidic Nanocarriers and Their Cutting-Edge Biomedical Applications
- PMID: 38613498
- PMCID: PMC11110070
- DOI: 10.1021/acsabm.4c00153
Liposomes to Cubosomes: The Evolution of Lipidic Nanocarriers and Their Cutting-Edge Biomedical Applications
Abstract
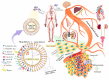
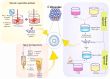
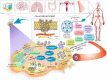
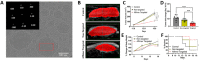
Lipidic nanoparticles have undergone extensive research toward the exploration of their diverse therapeutic applications. Although several liposomal formulations are in the clinic (e.g., DOXIL) for cancer therapy, there are many challenges associated with traditional liposomes. To address these issues, modifications in liposomal structure and further functionalization are desirable, leading to the emergence of solid lipid nanoparticles and the more recent liquid lipid nanoparticles. In this context, "cubosomes", third-generation lipidic nanocarriers, have attracted significant attention due to their numerous advantages, including their porous structure, structural adaptability, high encapsulation efficiency resulting from their extensive internal surface area, enhanced stability, and biocompatibility. Cubosomes offer the potential for both enhanced cellular uptake and controlled release of encapsulated payloads. Beyond cancer therapy, cubosomes have demonstrated effectiveness in wound healing, antibacterial treatments, and various dermatological applications. In this review, the authors provide an overview of the evolution of lipidic nanocarriers, spanning from conventional liposomes to solid lipid nanoparticles, with a special emphasis on the development and application of cubosomes. Additionally, it delves into recent applications and preclinical trials associated with cubosome formulations, which could be of significant interest to readers from backgrounds in nanomedicine and clinicians.
Keywords: cancer therapeutic; cubosomes; lipidic nanoparticles; liposomes; solid lipid nanoparticles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Comparison of cubosomes and liposomes for the encapsulation and delivery of curcumin.Soft Matter. 2021 Mar 28;17(12):3306-3313. doi: 10.1039/d0sm01655a. Epub 2021 Feb 24. Soft Matter. 2021. PMID: 33623948
-
Vesicular approach of cubosomes, its components, preparation techniques, evaluation and their appraisal for targeting cancer cells.J Liposome Res. 2024 Jun;34(2):368-384. doi: 10.1080/08982104.2023.2272643. Epub 2023 Oct 24. J Liposome Res. 2024. PMID: 37873797 Review.
-
Cubosomes: Versatile Nanosized Formulation for Efficient Delivery of Therapeutics.Curr Drug Deliv. 2022;19(6):644-657. doi: 10.2174/1567201818666210708123855. Curr Drug Deliv. 2022. PMID: 34238187 Review.
-
Cubosomes as versatile lipid nanocarriers for neurological disorder therapeutics: a comprehensive review.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jun;397(6):3729-3746. doi: 10.1007/s00210-023-02879-7. Epub 2023 Dec 14. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38095651 Review.
-
Liquid Crystalline Lipid Nanoparticles: Emerging Trends and Applications in Skin Cancer.Pharm Nanotechnol. 2024 Aug 30. doi: 10.2174/0122117385312450240816055942. Online ahead of print. Pharm Nanotechnol. 2024. PMID: 39219426
Cited by
-
Pre-clinical evaluation of a divalent liposomal vaccine to control invasive candidiasis.NPJ Vaccines. 2025 Jun 13;10(1):124. doi: 10.1038/s41541-025-01183-0. NPJ Vaccines. 2025. PMID: 40514358 Free PMC article.
-
Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments.Gels. 2025 Mar 26;11(4):245. doi: 10.3390/gels11040245. Gels. 2025. PMID: 40277680 Free PMC article. Review.
References
-
- Almeida L. N.; Araújo T. G. Solid lipid nanoparticles: the efficiency carrier for topical delivery of hydrophilic drugs. World J. Pharm. Pharm. Sci. 2017, 6, 175–189. 10.20959/wjpps20179-10061. - DOI
-
- Lopes R. M.; Gaspar M. M.; Pereira J.; Eleutério C. V.; Carvalheiro M.; Almeida A. J.; Cruz M. E. M. Liposomes versus lipid nanoparticles: comparative study of lipid-based systems as oryzalin carriers for the treatment of leishmaniasis. Journal of biomedical nanotechnology 2014, 10 (12), 3647–3657. 10.1166/jbn.2014.1874. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
