Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials
- PMID: 38613667
- PMCID: PMC11153294
- DOI: 10.1007/s00125-024-06144-1
Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials
Abstract
Aims/hypothesis: We conducted a systematic review and network meta-analysis to compare the efficacy and safety of s.c. administered tirzepatide vs s.c. administered semaglutide for adults of both sexes with type 2 diabetes mellitus.
Methods: We searched PubMed and Cochrane up to 11 November 2023 for RCTs with an intervention duration of at least 12 weeks assessing s.c. tirzepatide at maintenance doses of 5 mg, 10 mg or 15 mg once weekly, or s.c. semaglutide at maintenance doses of 0.5 mg, 1.0 mg or 2.0 mg once weekly, in adults with type 2 diabetes, regardless of background glucose-lowering treatment. Eligible trials compared any of the specified doses of tirzepatide and semaglutide against each other, placebo or other glucose-lowering drugs. Primary outcomes were changes in HbA1c and body weight from baseline. Secondary outcomes were achievement of HbA1c target of ≤48 mmol/mol (≤6.5%) or <53 mmol/mol (<7.0%), body weight loss of at least 10%, and safety outcomes including gastrointestinal adverse events and severe hypoglycaemia. We used version 2 of the Cochrane risk-of-bias tool (ROB 2) to assess the risk of bias, conducted frequentist random-effects network meta-analyses and evaluated confidence in effect estimates utilising the Confidence In Network Meta-Analysis (CINeMA) framework.
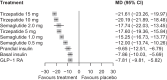
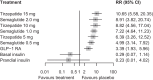
Results: A total of 28 trials with 23,622 participants (44.2% female) were included. Compared with placebo, tirzepatide 15 mg was the most efficacious treatment in reducing HbA1c (mean difference -21.61 mmol/mol [-1.96%]) followed by tirzepatide 10 mg (-20.19 mmol/mol [-1.84%]), semaglutide 2.0 mg (-17.74 mmol/mol [-1.59%]), tirzepatide 5 mg (-17.60 mmol/mol [-1.60%]), semaglutide 1.0 mg (-15.25 mmol/mol [-1.39%]) and semaglutide 0.5 mg (-12.00 mmol/mol [-1.09%]). In between-drug comparisons, all tirzepatide doses were comparable with semaglutide 2.0 mg and superior to semaglutide 1.0 mg and 0.5 mg. Compared with placebo, tirzepatide was more efficacious than semaglutide for reducing body weight, with reductions ranging from 9.57 kg (tirzepatide 15 mg) to 5.27 kg (tirzepatide 5 mg). Semaglutide had a less pronounced effect, with reductions ranging from 4.97 kg (semaglutide 2.0 mg) to 2.52 kg (semaglutide 0.5 mg). In between-drug comparisons, tirzepatide 15 mg, 10 mg and 5 mg demonstrated greater efficacy than semaglutide 2.0 mg, 1.0 mg and 0.5 mg, respectively. Both drugs increased incidence of gastrointestinal adverse events compared with placebo, while neither tirzepatide nor semaglutide increased the risk of serious adverse events or severe hypoglycaemia.
Conclusions/interpretation: Our data show that s.c. tirzepatide had a more pronounced effect on HbA1c and weight reduction compared with s.c. semaglutide in people with type 2 diabetes. Both drugs, particularly higher doses of tirzepatide, increased gastrointestinal adverse events.
Registration: PROSPERO registration no. CRD42022382594.
Keywords: GIP/GLP-1 receptor agonist; GLP-1 receptor agonist; Network meta-analysis; Semaglutide; Systematic review; Tirzepatide.
© 2024. The Author(s).
Figures

References
-
- Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2022;65(12):1925–1966. doi: 10.1007/S00125-022-05787-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

