Identification of the role of DAB2 and CXCL8 in uterine spiral artery remodeling in early-onset preeclampsia
- PMID: 38613672
- PMCID: PMC11016014
- DOI: 10.1007/s00018-024-05212-4
Identification of the role of DAB2 and CXCL8 in uterine spiral artery remodeling in early-onset preeclampsia
Abstract
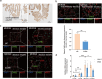
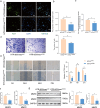
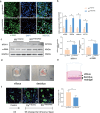
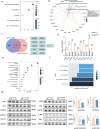
Aberrant remodeling of uterine spiral arteries (SPA) is strongly associated with the pathogenesis of early-onset preeclampsia (EOPE). However, the complexities of SPA transformation remain inadequately understood. We conducted a single-cell RNA sequencing analysis of whole placental tissues derived from patients with EOPE and their corresponding controls, identified DAB2 as a key gene of interest and explored the mechanism underlying the communication between Extravillous trophoblast cells (EVTs) and decidual vascular smooth muscle cells (dVSMC) through cell models and a placenta-decidua coculture (PDC) model in vitro. DAB2 enhanced the motility and viability of HTR-8/SVneo cells. After exposure to conditioned medium (CM) from HTR-8/SVneoshNC cells, hVSMCs exhibited a rounded morphology, indicative of dedifferentiation, while CM-HTR-8/SVneoshDAB2 cells displayed a spindle-like morphology. Furthermore, the PDC model demonstrated that CM-HTR-8/SVneoshDAB2 was less conducive to vascular remodeling. Further in-depth mechanistic investigations revealed that C-X-C motif chemokine ligand 8 (CXCL8, also known as IL8) is a pivotal regulator governing the dedifferentiation of dVSMC. DAB2 expression in EVTs is critical for orchestrating the phenotypic transition and motility of dVSMC. These processes may be intricately linked to the CXCL8/PI3K/AKT pathway, underscoring its central role in intricate SPA remodeling.
Keywords: CXCL8; DAB2; Early-onset preeclampsia; Spiral artery remodeling.
© 2024. The Author(s).
Conflict of interest statement
There are no competing financial or nonfinancial interests regarding this work.
Figures


Similar articles
-
ELABELA targets the MEK/ERK axis to enhance trophoblast invasion in early-onset preeclampsia.Sci Rep. 2025 Aug 11;15(1):29357. doi: 10.1038/s41598-025-15533-4. Sci Rep. 2025. PMID: 40789940 Free PMC article.
-
LncRNA-ATB Contributes to Severe Preeclampsia by Modulating the p53/MDM2 Pathway via PABPC1.FASEB J. 2025 Jun 30;39(12):e70736. doi: 10.1096/fj.202500351R. FASEB J. 2025. PMID: 40558399
-
Insufficient expression of VTN promotes the development of early-onset severe preeclampsia through the HEY1/autophagy signaling pathway.Placenta. 2025 Aug;168:56-66. doi: 10.1016/j.placenta.2025.06.011. Epub 2025 Jun 11. Placenta. 2025. PMID: 40516241
-
Gap junction connexins in female reproductive organs: implications for women's reproductive health.Hum Reprod Update. 2015 May-Jun;21(3):340-52. doi: 10.1093/humupd/dmv007. Epub 2015 Feb 9. Hum Reprod Update. 2015. PMID: 25667189
-
Accuracy of placental growth factor alone or in combination with soluble fms-like tyrosine kinase-1 or maternal factors in detecting preeclampsia in asymptomatic women in the second and third trimesters: a systematic review and meta-analysis.Am J Obstet Gynecol. 2023 Sep;229(3):222-247. doi: 10.1016/j.ajog.2023.03.032. Epub 2023 Mar 28. Am J Obstet Gynecol. 2023. PMID: 36990308
Cited by
-
Targeting A2M-LRP1 reverses uterine spiral artery remodeling disorder and alleviates the progression of preeclampsia.Cell Commun Signal. 2025 Feb 24;23(1):107. doi: 10.1186/s12964-025-02060-y. Cell Commun Signal. 2025. PMID: 39994728 Free PMC article.
-
A Multi-Algorithm Machine Learning Model for Predicting the Risk of Preterm Birth in Patients with Early-Onset Preeclampsia.Int J Gen Med. 2025 Aug 4;18:4195-4207. doi: 10.2147/IJGM.S521763. eCollection 2025. Int J Gen Med. 2025. PMID: 40786956 Free PMC article.
-
NFĸB and its inhibitors in preeclampsia: mechanisms and potential interventions.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 29. doi: 10.1007/s00210-025-04211-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40299024 Review.
-
Human Extravillous Trophoblasts Require SRC-2 for Sustained Viability, Migration, and Invasion.Cells. 2025 Jul 4;14(13):1024. doi: 10.3390/cells14131024. Cells. 2025. PMID: 40643542 Free PMC article.
-
The prediction of pre-eclampsia by using high resolution flow ultrasonography in determination vascularization of placental villi in late pregnancy.Sci Rep. 2025 Jul 4;15(1):23865. doi: 10.1038/s41598-025-09094-9. Sci Rep. 2025. PMID: 40615504 Free PMC article.
References
-
- Graham JB, Christopher WR, James MR, Ashley M. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. - PubMed
MeSH terms
Substances
Grants and funding
- 81830045/Innovative Research Group Project of the National Natural Science Foundation of China
- 82071652/Innovative Research Group Project of the National Natural Science Foundation of China
- 81971414/Innovative Research Group Project of the National Natural Science Foundation of China
- 81730040/Innovative Research Group Project of the National Natural Science Foundation of China
- 2022YFC2704500/Key Technologies Research and Development Program
LinkOut - more resources
Full Text Sources

