Advanced therapies in Parkinson's disease: an individualized approach to their indication
- PMID: 38613674
- PMCID: PMC11502575
- DOI: 10.1007/s00702-024-02773-3
Advanced therapies in Parkinson's disease: an individualized approach to their indication
Abstract
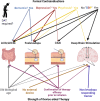
Device aided therapies (DAT) comprising the intrajejunal administration of levodopa/carbidopa intestinal gel (LCIG) and levodopa/carbidopa/entacapone intestinal gel (LECIG), the continuous subcutaneous application of foslevodopa/foscarbidopa or apomorphine infusion (CSAI) and deep brain stimulation (DBS) are used to treat Parkinson's disease with insufficient symptom alleviation under intensified pharmacotherapy. These DAT significantly differ in their efficacy profiles, indication, invasiveness, contraindications, and potential side effects. Usually, the evaluation of all these procedures is conducted simultaneously at the same point in time. However, as disease progression and symptom burden is extremely heterogeneous, clinical experience shows that patients reach the individual milestones for a certain therapy at different points in their disease course. Therefore, advocating for an individualized therapy evaluation for each DAT, requiring an ongoing evaluation. This necessitates that, during each consultation, the current symptomatology should be analyzed, and the potential suitability for a DAT be assessed. This work represents a critical interdisciplinary appraisal of these therapies in terms of their individual profiles and compares these DAT regarding contraindications, periprocedural considerations as well as their efficacy regarding motor- and non-motor deficits, supporting a personalized approach.
Keywords: CSAI; DBS; Device aided therapies; LCIG; Parkinson’s disease.
© 2024. The Author(s).
Figures
References
-
- Antonini A, Poewe W, Chaudhuri KR et al (2017) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20. 10.1016/j.parkreldis.2017.09.018 - PubMed
-
- Antonini A, Moro E, Godeiro C, Reichmann H (2018) Medical and surgical management of advanced Parkinson’s disease. Mov Disord 33:900–908. 10.1002/mds.27340 - PubMed
-
- Antonini A, Odin P, Schmidt P et al (2021) Validation and clinical value of the MANAGE-PD tool: a clinician-reported tool to identify Parkinson’s disease patients inadequately controlled on oral medications. Parkinsonism Relat Disord 92:59–66. 10.1016/j.parkreldis.2021.10.009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

