The nociceptive activity of peripheral sensory neurons is modulated by the neuronal membrane proteasome
- PMID: 38614084
- PMCID: PMC11157458
- DOI: 10.1016/j.celrep.2024.114058
The nociceptive activity of peripheral sensory neurons is modulated by the neuronal membrane proteasome
Abstract
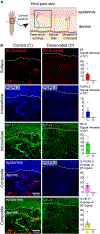
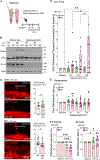
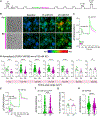
Proteasomes are critical for peripheral nervous system (PNS) function. Here, we investigate mammalian PNS proteasomes and reveal the presence of the neuronal membrane proteasome (NMP). We show that specific inhibition of the NMP on distal nerve fibers innervating the mouse hind paw leads to reduction in mechanical and pain sensitivity. Through investigating PNS NMPs, we demonstrate their presence on the somata and proximal and distal axons of a subset of dorsal root ganglion (DRG) neurons. Single-cell RNA sequencing experiments reveal that the NMP-expressing DRGs are primarily MrgprA3+ and Cysltr2+. NMP inhibition in DRG cultures leads to cell-autonomous and non-cell-autonomous changes in Ca2+ signaling induced by KCl depolarization, αβ-meATP, or the pruritogen histamine. Taken together, these data support a model whereby NMPs are expressed on a subset of somatosensory DRGs to modulate signaling between neurons of distinct sensory modalities and indicate the NMP as a potential target for controlling pain.
Keywords: CP: Molecular biology; CP: Neuroscience; DRG; neuronal membrane proteasome; neuronal signaling; peripheral nervous system; sensory behavior; sensory neurons.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, Marmiroli P, Bossi M, Oggioni N, D’Incalci M, and De Coster R (2007). Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp. Neurol. 204, 317–325. 10.1016/j.expneurol.2006.11.010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

