Tafamidis 61 mg Patient Characteristics and Persistency? A Retrospective Analysis of German Statutory Health Insurance Data (IQVIA™ LRx)
- PMID: 38615093
- PMCID: PMC11093959
- DOI: 10.1007/s40119-024-00365-6
Tafamidis 61 mg Patient Characteristics and Persistency? A Retrospective Analysis of German Statutory Health Insurance Data (IQVIA™ LRx)
Abstract
Introduction: Tafamidis is the first drug approved by the European Commission for the treatment of wild-type or hereditary transthyretin amyloid cardiomyopathy (ATTR-CM) in adults to reduce cardiovascular mortality and cardiovascular-related hospitalization. Real-world treatment patterns of tafamidis 61 mg in Germany are not well studied in patients with ATTR-CM.
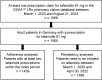
Methods: This was a non-interventional, retrospective, observational cohort study of adult patients in Germany based on the IQVIA pharmacy claims database (IQVIA™ LRx). Patients included in the analysis were statutory insured and received at least one prescription of tafamidis 61 mg between March 1, 2020 and August 31, 2022. Treatment adherence was analyzed using the modified medical possession ratio (mMPR) and proportion of days covered (PDC).
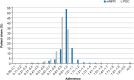
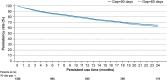
Results: Overall, 1565 adult patients received at least one tafamidis prescription in the study period. Their mean age was 78.3 years, 82.4% were male, and 23.2% were treated by a cardiologist. Persistency rates for patients treated with tafamidis 61 mg were high: 78.0% for 12 months and 65.1% for 24 months after treatment initiation. Patients also had high adherence rate on filling their prescriptions on time: 94.6% and 90.5% of patients had adherence rates of at least 80%, measured by mMPR and PDC, respectively.
Conclusions: In the IQVIA™ LRx database, patients prescribed tafamidis 61 mg in Germany displayed high adherence and persistency rates, which suggest good drug tolerability and ease of use.
Keywords: ATTR-CM; Adherence; Persistence; Tafamidis.
© 2024. The Author(s).
Conflict of interest statement
Sepideh Attal, Jason Kemner, Jose Alvir, are employees of Pfizer and own stock and/or stock options. Sebastian Barth is an employee of IQVIA Commercial GmbH & Co. OHG, which received funding from Pfizer to assist in the conduct of this study and for manuscript development. Sofia Schuessler worked at IQVIA GmbH & Co. OHG at the time of the study and was a paid consultant to Pfizer in connection with this study.
Figures
Comment in
-
Letter to the Editor Regarding 'Tafamidis 61 mg Patient Characteristics and Persistency? A Retrospective Analysis of German Statutory Health Insurance Data (IQVIA™ LRx)'.Cardiol Ther. 2024 Dec;13(4):811-814. doi: 10.1007/s40119-024-00382-5. Epub 2024 Oct 23. Cardiol Ther. 2024. PMID: 39441481 Free PMC article. No abstract available.
References
-
- Lachmann HJ, Hawkins PN. Systemic amyloidosis. Curr Opin Pharmacol. 2006;6:214–20. 10.1016/j.coph.2005.10.005. - PubMed
-
- Sipe JD, Benson MD, Buxbaum JN, et al. Nomenclature 2014: amyloid fibril proteins and clinical classification of the amyloidosis. Amyloid. 2014;21:221–4. 10.3109/13506129.2014.964858. - PubMed
-
- Dubrey SW, Hawkins PN, Falk RH. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97:75–84. 10.1136/hrt.2009.190405. - PubMed
-
- Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–16. 10.1056/NEJMoa1805689. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials