Effects of semaglutide-loaded lipid nanocapsules on metabolic dysfunction-associated steatotic liver disease
- PMID: 38615156
- PMCID: PMC11385015
- DOI: 10.1007/s13346-024-01576-z
Effects of semaglutide-loaded lipid nanocapsules on metabolic dysfunction-associated steatotic liver disease
Abstract
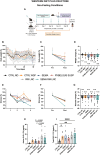
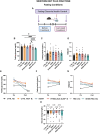
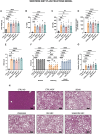
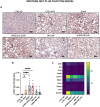
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a highly prevalent chronic liver disease that can progress to end-stage conditions with life-threatening complications, but no pharmacologic therapy has been approved. Drug delivery systems such as lipid nanocapsules (LNC) are very versatile platforms that are easy to produce and can induce the secretion of the native glucagon-like peptide 1 (GLP-1) when orally administered. GLP-1 analogs are currently being studied in clinical trials in the context of MASLD. Our nanosystem provides with increased levels of the native GLP-1 and increased plasmatic absorption of the encapsulated GLP-1 analog (semaglutide). Our goal was to use our strategy to demonstrate a better outcome and a greater impact on the metabolic syndrome associated with MASLD and on liver disease progression with our strategy compared with the oral marketed version of semaglutide, Rybelsus®. Therefore, we studied the effect of our nanocarriers on a dietary mouse model of MASLD, the Western diet model, during a daily chronic treatment of 4 weeks. Overall, the results showed a positive impact of semaglutide-loaded lipid nanocapsules towards the normalization of glucose homeostasis and insulin resistance. In the liver, there were no significant changes in lipid accumulation, but an improvement in markers related to inflammation was observed. Overall, our strategy had a positive trend on the metabolic syndrome and at reducing inflammation, mitigating the progression of the disease. Oral administration of the nanosystem was more efficient at preventing the progression of the disease to more severe states when compared to the administration of Rybelsus®, as a suspension.
Keywords: GLP-1 analogs; MASH; MASLD; Rybelsus; Semaglutide.
© 2024. The Author(s).
Conflict of interest statement
A.B. is inventor of a related patent application (WO/2020/254083A1 - Lipid nanocapsules charged with incretin mimetics).
Figures
Similar articles
-
Exploiting the biological effect exerted by lipid nanocapsules in non-alcoholic fatty liver disease.J Control Release. 2023 Apr;356:542-553. doi: 10.1016/j.jconrel.2023.03.012. Epub 2023 Mar 15. J Control Release. 2023. PMID: 36907563 Free PMC article.
-
Semaglutide Improves Liver Steatosis and De Novo Lipogenesis Markers in Obese and Type-2-Diabetic Mice with Metabolic-Dysfunction-Associated Steatotic Liver Disease.Int J Mol Sci. 2024 Mar 4;25(5):2961. doi: 10.3390/ijms25052961. Int J Mol Sci. 2024. PMID: 38474208 Free PMC article.
-
Glucagon-like peptide 1 agonists are potentially useful drugs for treating metabolic dysfunction-associated steatotic liver disease.World J Gastroenterol. 2024 Aug 14;30(30):3541-3547. doi: 10.3748/wjg.v30.i30.3541. World J Gastroenterol. 2024. PMID: 39193573 Free PMC article.
-
The role of glucagon-like peptide-1 receptor agonists in metabolic dysfunction-associated steatohepatitis.Diabetes Obes Metab. 2024 Jun;26(6):2001-2016. doi: 10.1111/dom.15524. Epub 2024 Mar 21. Diabetes Obes Metab. 2024. PMID: 38511418 Review.
-
Oral semaglutide in type 2 diabetes.J Diabetes Complications. 2020 Apr;34(4):107520. doi: 10.1016/j.jdiacomp.2019.107520. Epub 2020 Jan 8. J Diabetes Complications. 2020. PMID: 31952996 Review.
Cited by
-
Gut hormone stimulation as a therapeutic approach in oral peptide delivery.J Control Release. 2024 Sep;373:31-37. doi: 10.1016/j.jconrel.2024.07.007. Epub 2024 Jul 8. J Control Release. 2024. PMID: 38971429 Free PMC article.
-
Advances in Oral Biomacromolecule Therapies for Metabolic Diseases.Pharmaceutics. 2025 Feb 12;17(2):238. doi: 10.3390/pharmaceutics17020238. Pharmaceutics. 2025. PMID: 40006605 Free PMC article. Review.
-
Smart control lipid-based nanocarriers for fine-tuning gut hormone secretion.Sci Adv. 2024 Dec 13;10(50):eadq9909. doi: 10.1126/sciadv.adq9909. Epub 2024 Dec 13. Sci Adv. 2024. PMID: 39671480 Free PMC article.
References
-
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne C, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer D, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot B, Korenjak M, Kowdley K, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell E, Roden M, Romero-Gomez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN, NNC Group. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–86. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

