Surface-Functionalized Microgels as Artificial Antigen-Presenting Cells to Regulate Expansion of T Cells
- PMID: 38615189
- PMCID: PMC11293993
- DOI: 10.1002/adma.202309860
Surface-Functionalized Microgels as Artificial Antigen-Presenting Cells to Regulate Expansion of T Cells
Abstract
Artificial antigen-presenting cells (aAPCs) are currently used to manufacture T cells for adoptive therapy in cancer treatment, but a readily tunable and modular system can enable both rapid T cell expansion and control over T cell phenotype. Here, it is shown that microgels with tailored surface biochemical properties can serve as aAPCs to mediate T cell activation and expansion. Surface functionalization of microgels is achieved via layer-by-layer coating using oppositely charged polymers, forming a thin but dense polymer layer on the surface. This facile and versatile approach is compatible with a variety of coating polymers and allows efficient and flexible surface-specific conjugation of defined peptides or proteins. The authors demonstrate that tethering appropriate stimulatory ligands on the microgel surface efficiently activates T cells for polyclonal and antigen-specific expansion. The expansion, phenotype, and functional outcome of primary mouse and human T cells can be regulated by modulating the concentration, ratio, and distribution of stimulatory ligands presented on microgel surfaces as well as the stiffness and viscoelasticity of the microgels.
Keywords: T cell activation; antigen‐specific T cell expansion; granular hydrogels; microgels; surface functionalization; viscoelasticity.
© 2024 Wiley‐VCH GmbH.
Figures




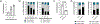
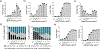
References
-
- Huppa JB, Davis MM, Nat. Rev. Immunol. 2003, 3, 973–983. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

