Redox regulation of macrophages
- PMID: 38615489
- PMCID: PMC11026845
- DOI: 10.1016/j.redox.2024.103123
Redox regulation of macrophages
Abstract
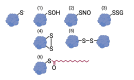
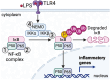
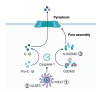
Redox signaling, a mode of signal transduction that involves the transfer of electrons from a nucleophilic to electrophilic molecule, has emerged as an essential regulator of inflammatory macrophages. Redox reactions are driven by reactive oxygen/nitrogen species (ROS and RNS) and redox-sensitive metabolites such as fumarate and itaconate, which can post-translationally modify specific cysteine residues in target proteins. In the past decade our understanding of how ROS, RNS, and redox-sensitive metabolites control macrophage function has expanded dramatically. In this review, we discuss the latest evidence of how ROS, RNS, and metabolites regulate macrophage function and how this is dysregulated with disease. We highlight the key tools to assess redox signaling and important questions that remain.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures


References
-
- McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

