Urolithin A inhibits breast cancer progression via activating TFEB-mediated mitophagy in tumor macrophages
- PMID: 38615740
- PMCID: PMC11954813
- DOI: 10.1016/j.jare.2024.04.010
Urolithin A inhibits breast cancer progression via activating TFEB-mediated mitophagy in tumor macrophages
Erratum in
-
Corrigendum to "Urolithin A inhibits breast cancer progression via activating TFEB-mediated mitophagy in tumor macrophages" [J. Adv. Res. 69 (2025) 125-138].J Adv Res. 2025 Jul;73:765-767. doi: 10.1016/j.jare.2025.05.012. Epub 2025 May 10. J Adv Res. 2025. PMID: 40350365 Free PMC article. No abstract available.
Abstract
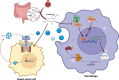
Introduction: Urolithin A (UA) is a naturally occurring compound that is converted from ellagitannin-like precursors in pomegranates and nuts by intestinal flora. Previous studies have found that UA exerts tumor-suppressive effects through antitumor cell proliferation and promotion of memory T-cell expansion, but its role in tumor-associated macrophages remains unknown.
Objectives: Our study aims to reveal how UA affects tumor macrophages and tumor cells to inhibit breast cancer progression.
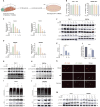
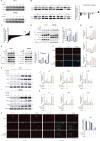
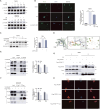
Methods: Observe the effect of UA treatment on breast cancer progression though in vivo and in vitro experiments. Western blot and PCR assays were performed to discover that UA affects tumor macrophage autophagy and inflammation. Co-ip and Molecular docking were used to explore specific molecular mechanisms.
Results: We observed that UA treatment could simultaneously inhibit harmful inflammatory factors, especially for InterleuKin-6 (IL-6) and tumor necrosis factor α (TNF-α), in both breast cancer cells and tumor-associated macrophages, thereby improving the tumor microenvironment and delaying tumor progression. Mechanistically, UA induced the key regulator of autophagy, transcription factor EB (TFEB), into the nucleus in a partially mTOR-dependent manner and inhibited the ubiquitination degradation of TFEB, which facilitated the clearance of damaged mitochondria via the mitophagy-lysosomal pathway in macrophages under tumor supernatant stress, and reduced the deleterious inflammatory factors induced by the release of nucleic acid from damaged mitochondria. Molecular docking and experimental studies suggest that UA block the recognition of TFEB by 1433 and induce TFEB nuclear localization. Notably, UA treatment demonstrated inhibitory effects on tumor progression in multiple breast cancer models.
Conclusion: Our study elucidated the anti-breast cancer effect of UA from the perspective of tumor-associated macrophages. Specifically, TFEB is a crucial downstream target in macrophages.
Keywords: Breast cancer; Mitophagy; TFEB; Tumor-associated macrophages; Urolithin A.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Singh A., D’Amico D., Andreux P.A., Fouassier A.M., Blanco-Bose W., Evans M., et al. Urolithin a improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100633. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

