Defining Ultra-Massive Transfusion through a Systematic Review
- PMID: 38616968
- PMCID: PMC11008908
- DOI: 10.1016/j.amjsurg.2023.09.024
Defining Ultra-Massive Transfusion through a Systematic Review
Abstract
Background: Despite the widespread use of ultra-massive transfusion (UMT) as an intervention for trauma patients in hemorrhagic shock, no standard definition exists. We performed a systematic review to determine a consensus definition for UMT.
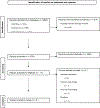
Methods: A search was performed from 1979-2022. The authors screened studies defining UMT and associated outcomes as defined by our prespecified PICO questions. The PRISMA guidelines were used.
Results: 1662 articles met criteria for eligibility assessment, 17 for full-text review and eight for data extraction. Only two studies demonstrated a consensus definition of UMT, which used ≥20 units of red blood cell product within 24hrs. Parameters associated with increased mortality included lower blood pressure, lower pulse and lower Glasgow Coma Score at the time of presentation and a higher injury severity score and undergoing a resuscitative thoracotomy.
Conclusions: The absence of a consensus definition for UMT raises challenges from clinical, research and ethical perspectives. Based on our findings, the authors advocate for the feasibility of standardizing the definition of UMT as ≥20 units of red blood cell product within 24hrs.
Keywords: Blood transfusion; consensus definition; systematic review; trauma patient; ultra-massive.
Conflict of interest statement
Conflict of Interest Statement: The authors have no conflicts of interest to disclose
Figures
References
-
- Thompson P, Strandenes G. (2020). The History of Fluid Resuscitation for Bleeding. In: Spinella P. (ed) Damage Control Resuscitation. Springer, Cham. 10.1007/978-3-030-20820-2_1 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous