This is a preprint.
Seq-Scope Protocol: Repurposing Illumina Sequencing Flow Cells for High-Resolution Spatial Transcriptomics
- PMID: 38617262
- PMCID: PMC11014489
- DOI: 10.1101/2024.03.29.587285
Seq-Scope Protocol: Repurposing Illumina Sequencing Flow Cells for High-Resolution Spatial Transcriptomics
Update in
-
Seq-Scope: repurposing Illumina sequencing flow cells for high-resolution spatial transcriptomics.Nat Protoc. 2025 Mar;20(3):643-689. doi: 10.1038/s41596-024-01065-0. Epub 2024 Oct 31. Nat Protoc. 2025. PMID: 39482362 Review.
Abstract
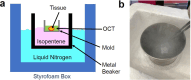
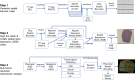
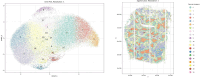
Spatial transcriptomics (ST) technologies represent a significant advance in gene expression studies, aiming to profile the entire transcriptome from a single histological slide. These techniques are designed to overcome the constraints faced by traditional methods such as immunostaining and RNA in situ hybridization, which are capable of analyzing only a few target genes simultaneously. However, the application of ST in histopathological analysis is also limited by several factors, including low resolution, a limited range of genes, scalability issues, high cost, and the need for sophisticated equipment and complex methodologies. Seq-Scope-a recently developed novel technology-repurposes the Illumina sequencing platform for high-resolution, high-content spatial transcriptome analysis, thereby overcoming these limitations. Here we provide a detailed step-by-step protocol to implement Seq-Scope with an Illumina NovaSeq 6000 sequencing flow cell that allows for the profiling of multiple tissue sections in an area of 7 mm × 7 mm or larger. In addition to detailing how to prepare a frozen tissue section for both histological imaging and sequencing library preparation, we provide comprehensive instructions and a streamlined computational pipeline to integrate histological and transcriptomic data for high-resolution spatial analysis. This includes the use of conventional software tools for single cell and spatial analysis, as well as our recently developed segmentation-free method for analyzing spatial data at submicrometer resolution. Given its adaptability across various biological tissues, Seq-Scope establishes itself as an invaluable tool for researchers in molecular biology and histology.
Conflict of interest statement
COMPETING INTERESTS HMK owns stock for Regeneron Pharmaceuticals. JHL is an inventor on a patent and pending patent applications related to Seq-Scope.
Figures

















References
-
- Sergio Marco S. et al. Optimizing Xenium In Situ data utility by quality assessment and best practice analysis workflows. bioRxiv, 2023.2002.2013.528102 (2023).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
