This is a preprint.
A disease-associated PPP2R3C-MAP3K1 phospho-regulatory module controls centrosome function
- PMID: 38617270
- PMCID: PMC11014585
- DOI: 10.1101/2024.04.02.587836
A disease-associated PPP2R3C-MAP3K1 phospho-regulatory module controls centrosome function
Update in
-
A disease-associated PPP2R3C-MAP3K1 phospho-regulatory module controls centrosome function.Curr Biol. 2024 Oct 21;34(20):4824-4834.e6. doi: 10.1016/j.cub.2024.08.058. Epub 2024 Sep 23. Curr Biol. 2024. PMID: 39317195
Abstract
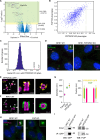
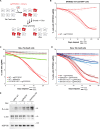
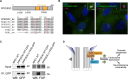
Centrosomes have critical roles in microtubule organization and in cell signaling.1-8 However, the mechanisms that regulate centrosome function are not fully defined, and thus how defects in centrosomal regulation contribute to disease is incompletely understood. From functional genomic analyses, we find here that PPP2R3C, a PP2A phosphatase subunit, is a distal centriole protein and functional partner of centriolar proteins CEP350 and FOP. We further show that a key function of PPP2R3C is to counteract the kinase activity of MAP3K1. In support of this model, MAP3K1 knockout suppresses growth defects caused by PPP2R3C inactivation, and MAP3K1 and PPP2R3C have opposing effects on basal and microtubule stress-induced JNK signaling. Illustrating the importance of balanced MAP3K1 and PPP2R3C activities, acute overexpression of MAP3K1 severely inhibits centrosome function and triggers rapid centriole disintegration. Additionally, inactivating PPP2R3C mutations and activating MAP3K1 mutations both cause congenital syndromes characterized by gonadal dysgenesis.9-15 As a syndromic PPP2R3C variant is defective in centriolar localization and binding to centriolar protein FOP, we propose that imbalanced activity of this centrosomal kinase-phosphatase pair is the shared cause of these disorders. Thus, our findings reveal a new centrosomal phospho-regulatory module, shed light on disorders of gonadal development, and illustrate the power of systems genetics to identify previously unrecognized gene functions.
Keywords: Centriole; PP2A; centrosome; cilia; functional genomics; gonadal dysgenesis; kinase; phosphatase.
Conflict of interest statement
Conflict of interest statement The authors declare no conflicts of interest
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
