This is a preprint.
Pseudomonas aeruginosa surface motility and invasion into competing communities enhances interspecies antagonism
- PMID: 38617332
- PMCID: PMC11014535
- DOI: 10.1101/2024.04.03.588010
Pseudomonas aeruginosa surface motility and invasion into competing communities enhances interspecies antagonism
Update in
-
Pseudomonas aeruginosa surface motility and invasion into competing communities enhance interspecies antagonism.mBio. 2024 Sep 11;15(9):e0095624. doi: 10.1128/mbio.00956-24. Epub 2024 Aug 6. mBio. 2024. PMID: 39105585 Free PMC article.
Abstract
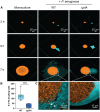
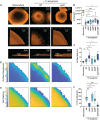
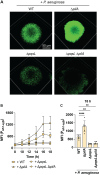
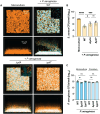
Chronic polymicrobial infections involving Pseudomonas aeruginosa and Staphylococcus aureus are prevalent, difficult to eradicate, and associated with poor health outcomes. Therefore, understanding interactions between these pathogens is important to inform improved treatment development. We previously demonstrated that P. aeruginosa is attracted to S. aureus using type IV pili-mediated chemotaxis, but the impact of attraction on S. aureus growth and physiology remained unknown. Using live single-cell confocal imaging to visualize microcolony structure, spatial organization, and survival of S. aureus during coculture, we found that interspecies chemotaxis provides P. aeruginosa a competitive advantage by promoting invasion into and disruption of S. aureus microcolonies. This behavior renders S. aureus susceptible to P. aeruginosa antimicrobials. Conversely, in the absence of type IV pilus motility, P. aeruginosa cells exhibit reduced invasion of S. aureus colonies. Instead, P. aeruginosa builds a cellular barrier adjacent to S. aureus and secretes diffusible, bacteriostatic antimicrobials like 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO) into the S. aureus colonies. P. aeruginosa reduced invasion leads to the formation of denser and thicker S. aureus colonies with significantly increased HQNO-mediated lactic acid fermentation, a physiological change that could complicate the effective treatment of infections. Finally, we show that P. aeruginosa motility modifications of spatial structure enhance competition against S. aureus. Overall, these studies build on our understanding of how P. aeruginosa type IV pili-mediated interspecies chemotaxis mediates polymicrobial interactions, highlighting the importance of spatial positioning in mixed-species communities.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; biofilms; polymicrobial; spatial organization; type IV pili motility.
Conflict of interest statement
COMPETING INTERESTS The authors have declared no competing interests.
Figures


References
-
- Strassmann J.E., Gilbert O.M., and Queller D.C., Kin discrimination and cooperation in microbes. Annu Rev Microbiol, 2011. 65: p. 349–67. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
